The preparation method of 2-chloro-4'-fluoroacetophenone
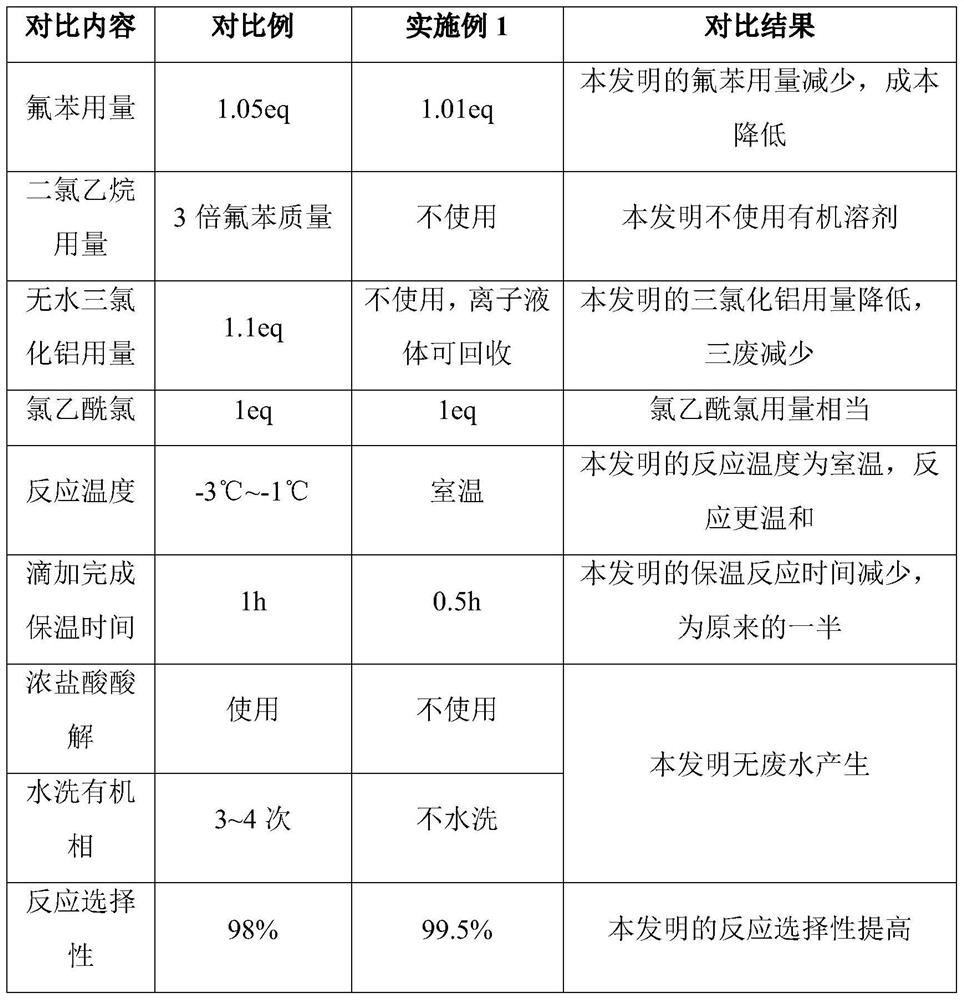
A technology of fluoroacetophenone and chlorination, which is applied in the field of preparation of 2-chloro-4'-fluoroacetophenone, which can solve the problem of high energy consumption, product irritation and safety, high environmental risk, and a large amount of aluminum trichloride Organic waste liquid and other problems, to achieve the effect of mild reaction conditions, green reaction, cost reduction and solid waste generation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0016] The preparation method of 2-chloro-4'-fluoroacetophenone comprises the following steps:
[0017] a. Add chloroacetyl chloride dropwise to fluorobenzene and ionic liquid at 0-30°C, and continue to react at 0-30°C after the addition is completed;
[0018] b. After the reaction is complete, distill to obtain 2-chloro-4'-fluoroacetophenone.
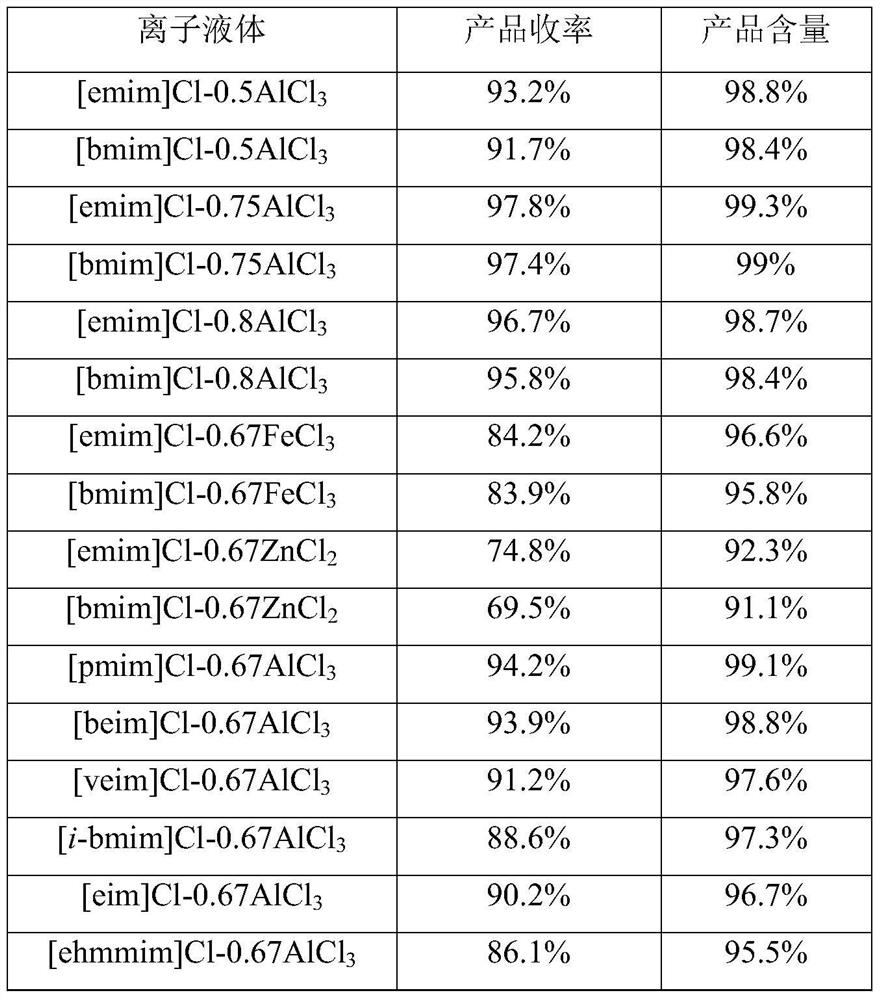
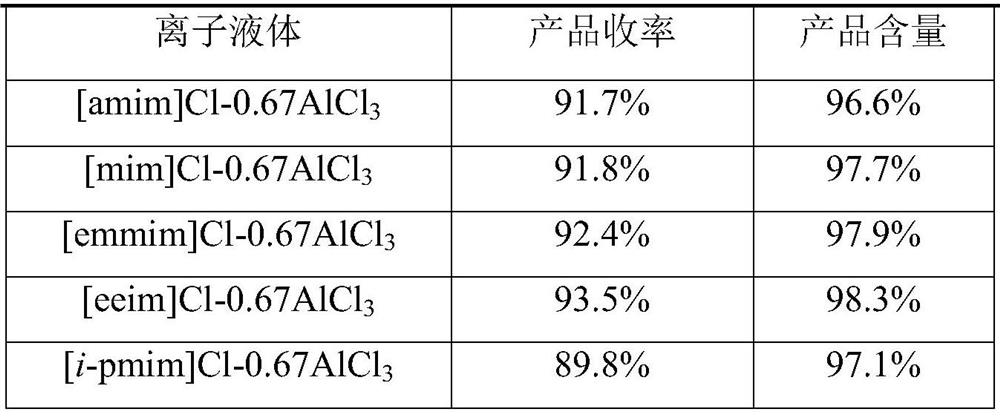
[0019] In the above-mentioned preparation method of 2-chloro-4'-fluoroacetophenone, the ionic liquid described in step a is an aluminum chloride-type ionic liquid. Described aluminum chloride type ionic liquid is [emim]Cl-0.5AlCl 3 、[emim]Cl-0.67AlCl 3 、[emim]Cl-0.75AlCl 3 、[emim]Cl-0.8AlCl 3 、[bmim]Cl-0.5AlCl 3 、[bmim]Cl-0.67AlCl 3 、[bmim]Cl-0.75AlCl 3 、[bmim]Cl-0.8AlCl 3 、[mmim]Cl-0.67AlCl 3 、[pmim]Cl-0.67AlCl 3 、[beim]Cl-0.67AlCl 3 , [veim]Cl-0.67AlCl 3 , [eim]Cl-0.67AlCl 3 、[amim]Cl-0.67AlCl 3 、[emmim]Cl-0.67AlCl 3 , [mim]Cl-0.67AlCl 3 or [eeim]Cl-0.67AlCl 3 any of the.
[0020] In the above preparation method of ...
Embodiment 1
[0028] Add 97g (1.01mol, 1.01eq) of fluorobenzene and 206.6g (0.5mol, 0.5eq) of ionic liquid [emim]Cl-0.67AlCl into a 500mL reaction flask 3 113 g (1 mol, 1 eq) of chloroacetyl chloride was added dropwise at room temperature, and stirring was continued for 30 minutes after the addition was completed. The central control fluorophenyl basically disappeared, and then the reaction solution was distilled under reduced pressure under a pressure of 10mmHg, and the fractions at 128°C to 132°C were collected to obtain 169.3g of 2-chloro-4'-fluoroacetophenone. The selectivity of the reaction 99.5%, content 99.5%, yield 98.1%.
Embodiment 2
[0030] Add 98g (1.02mol, 1.02eq) of fluorobenzene and 220.7g (0.5mol, 0.5eq) of ionic liquid [bmim]Cl-0.67AlCl into a 500mL reaction flask 3 , 113 g (1 mol, 1 eq) of chloroacetyl chloride was added dropwise at 0° C., and the stirring reaction was continued for 1.5 hours after the dropwise addition was completed. The central control fluorophenyl basically disappeared, the temperature was raised to 130°C, and 167.2g of 2-chloro-4'-fluoroacetophenone was obtained by distillation under reduced pressure under the condition of 10mmHg, the content was 99.2%, and the yield was 96.87%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com