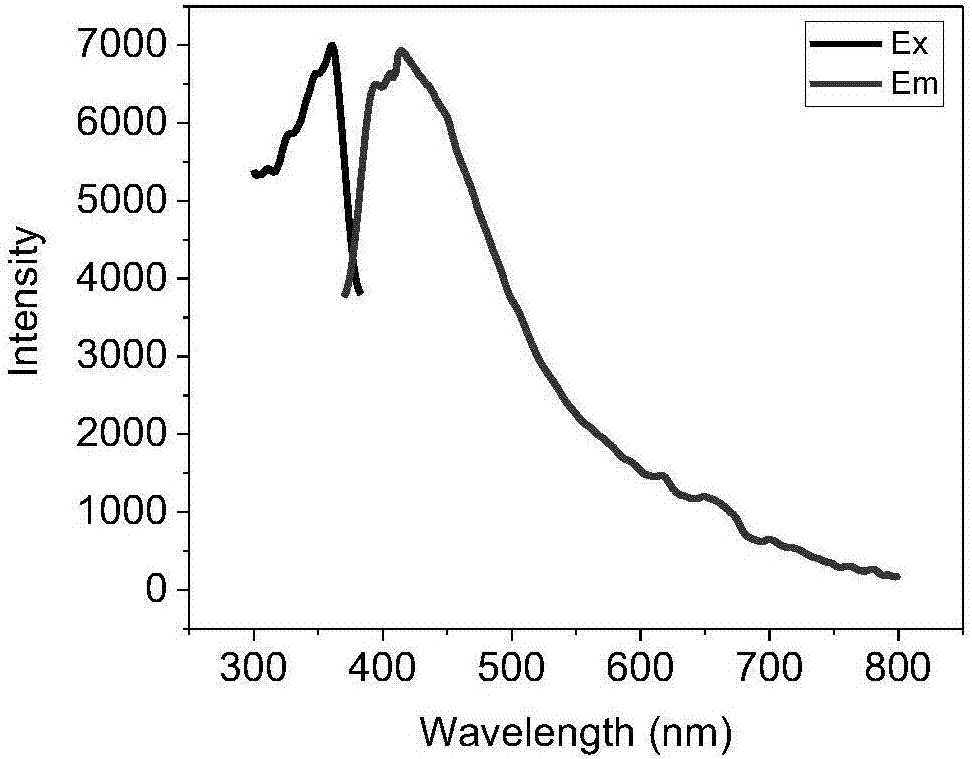
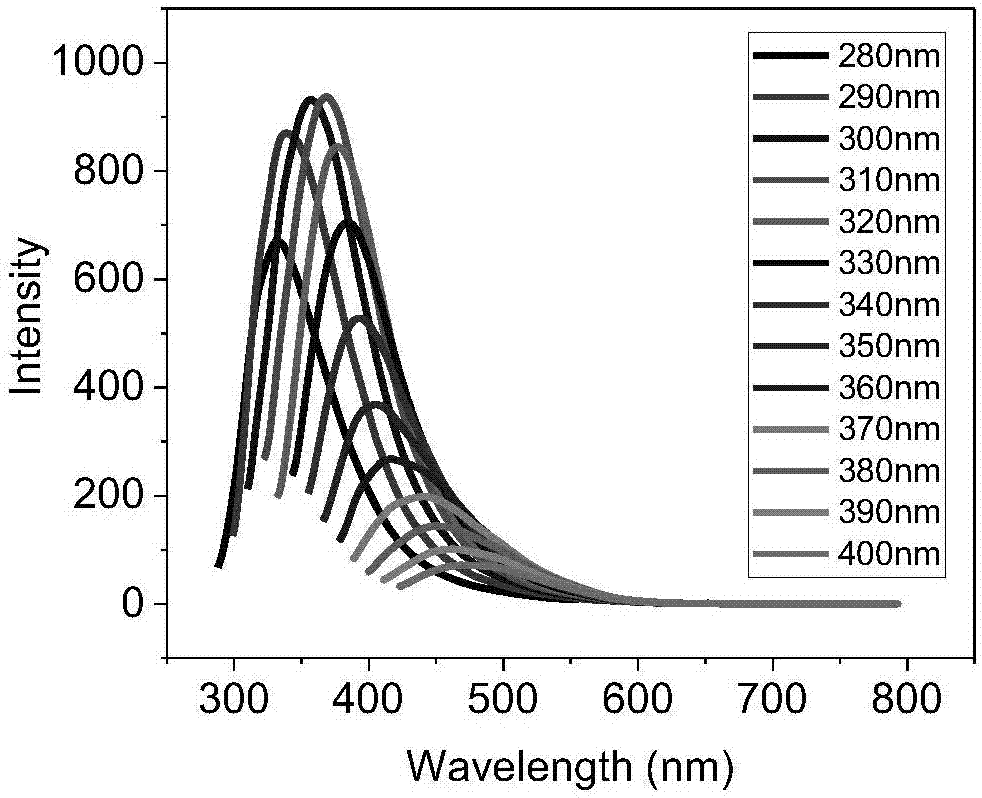
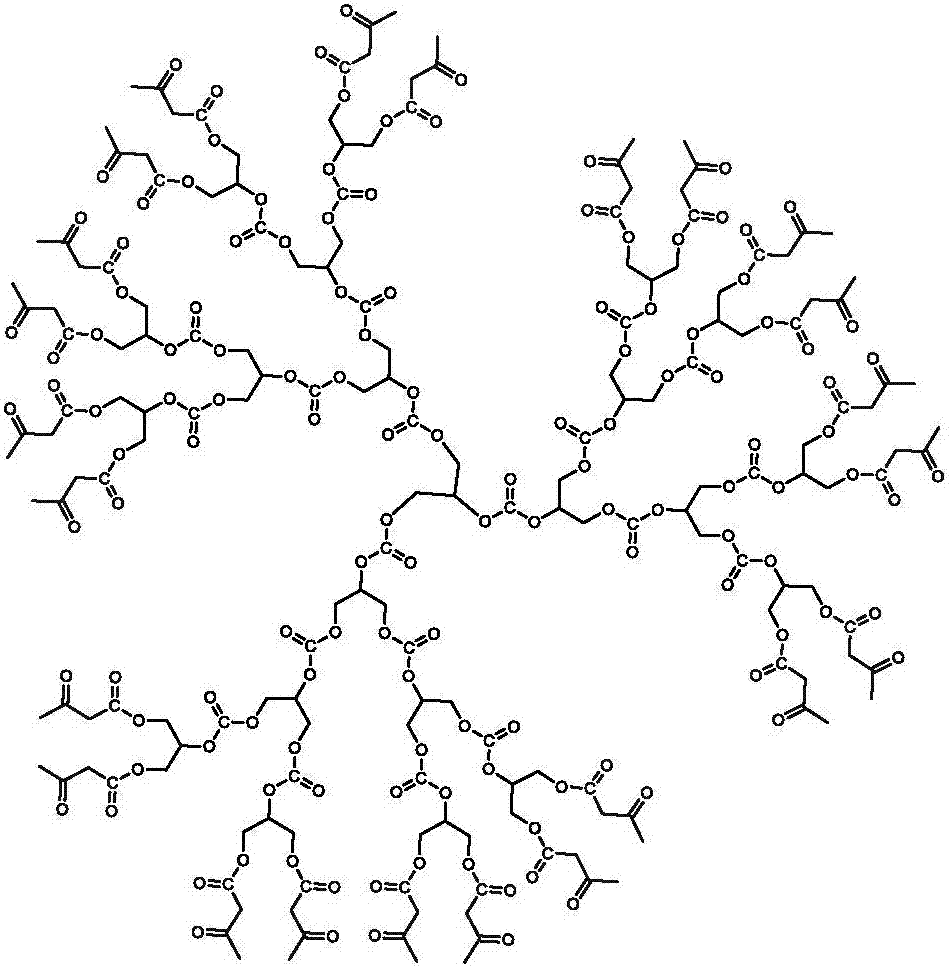
Carbonyl terminated hyperbranched polycarbonate capable of emitting bright fluorescence and preparation method thereof
A technology of polycarbonate and hydroxyl-terminated hyperbranching, which is applied in the field of hyperbranched polycarbonate and its preparation, can solve the problems of phosgene being highly toxic, hydrogen chloride is highly irritating, and environmental pollution, and achieves low cytotoxicity and less pollution from three wastes. , the effect of a wide range of applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0034] The first step, the preparation method of hydroxyl-terminated hyperbranched polycarbonate: under the protection of nitrogen, diethyl carbonate (0.126mol) and glycerol (0.151mol) are in a molar ratio of 1:1.2, heated and stirred at 90°C After 20 minutes, a catalyst, p-toluenesulfonic acid (0.29 g, accounting for 1% of the total mass of reactants), was added to gradually raise the temperature to 110° C., and reacted for 4 hours. Distill under reduced pressure to remove unreacted diethyl carbonate and cool to room temperature. Then the product was dissolved in water, poured into a dialysis bag and dialyzed for 48 hours to remove unreacted glycerin, oligomers and catalysts, and the solution in the inner layer of the dialysis bag was rotary evaporated and vacuum-dried to obtain a hydroxyl-terminated hyperbranched polymer Carbonate 19.87g, yield 87.4%.
[0035] Second step, the preparation method of the hyperbranched polycarbonate of carbonyl termination: under nitrogen prot...
example 2
[0037] The first step, the preparation method of hydroxyl-terminated hyperbranched polycarbonate: under the protection of nitrogen, diethyl carbonate (0.126mol) and glycerol (0.162mol) are in a molar ratio of 1:1.29, heated and stirred at 90°C After 40 minutes, a catalyst, p-toluenesulfonic acid (0.16 g, 0.5% of the total mass of reactants) was added and the temperature was gradually raised to 150° C., and the reaction was carried out for 7 hours. Distill under reduced pressure to remove unreacted diethyl carbonate and cool to room temperature. Then the product was dissolved in water, poured into a dialysis bag and dialyzed for 48 hours to remove unreacted glycerin, oligomers and catalysts, and the solution in the inner layer of the dialysis bag was rotary evaporated and vacuum-dried to obtain a hydroxyl-terminated hyperbranched polymer Carbonate 19.13g, yield 84.2%.
[0038]The second step, the preparation method of carbonyl-terminated hyperbranched polycarbonate: under nitr...
example 3
[0040] The first step, the preparation method of hydroxyl-terminated hyperbranched polycarbonate: under the protection of nitrogen, diethyl carbonate (0.126mol) and glycerol (0.189mol) are in a molar ratio of 1:1.5, heated and stirred at 80°C After 50 minutes, the catalyst p-toluenesulfonic acid (0.26 g, 0.8% of the total mass of the reactants) was added and the temperature was gradually raised to 140° C., and the reaction was carried out for 8 hours. Distill under reduced pressure to remove unreacted diethyl carbonate and cool to room temperature. Then the product was dissolved in water, poured into a dialysis bag and dialyzed for 48 hours to remove unreacted glycerin, oligomers and catalysts, and the solution in the inner layer of the dialysis bag was rotary evaporated and vacuum-dried to obtain a hydroxyl-terminated hyperbranched polymer Carbonate 20.54g, yield 90.4%.
[0041] Second step, the preparation method of the hyperbranched polycarbonate of carbonyl termination: u...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com