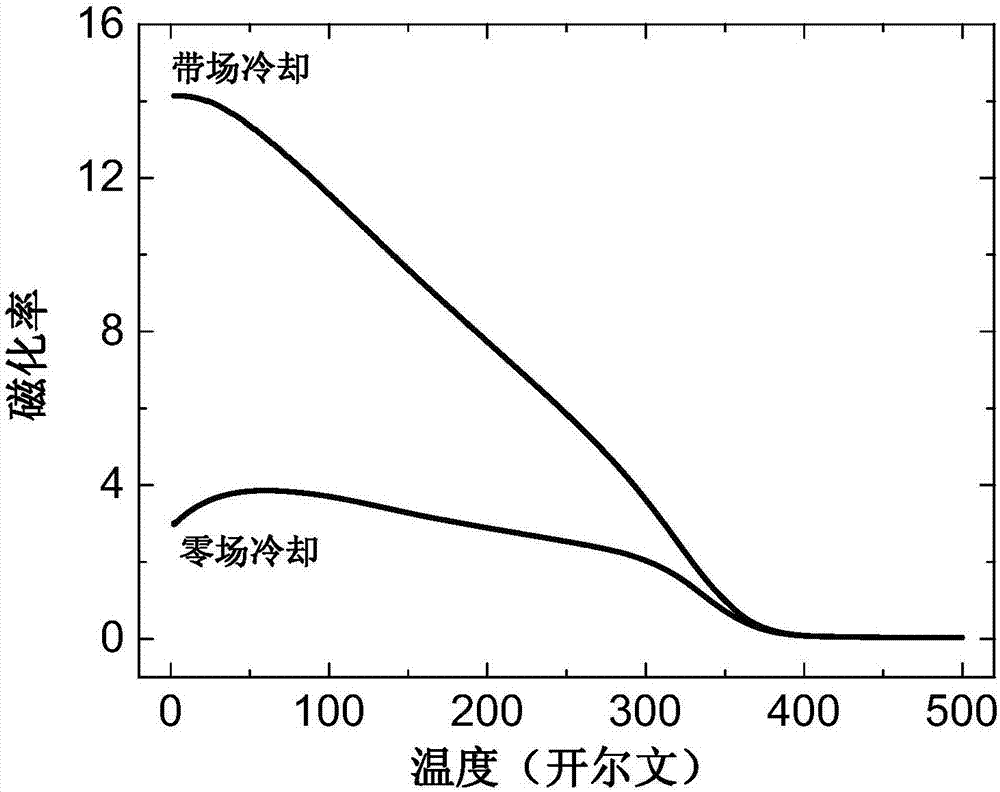
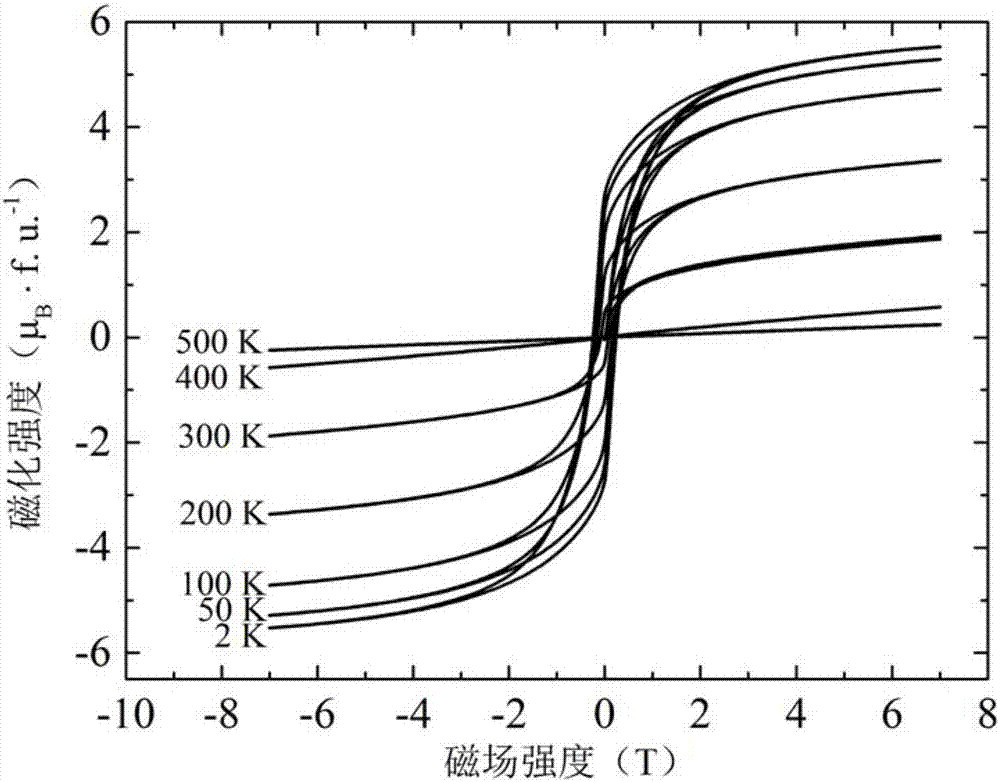
Ferrimagnetism semimetal NaCu3Fe2Os2O12 and preparation method thereof
A nacu3fe2os2o12, ferrimagnetic technology, applied in ruthenium/rhodium/palladium/osmium/iridium/platinum compounds, magnetic materials, magnetic objects, etc., can solve the problems of low spin-polarized electron efficiency and low spin-electron efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
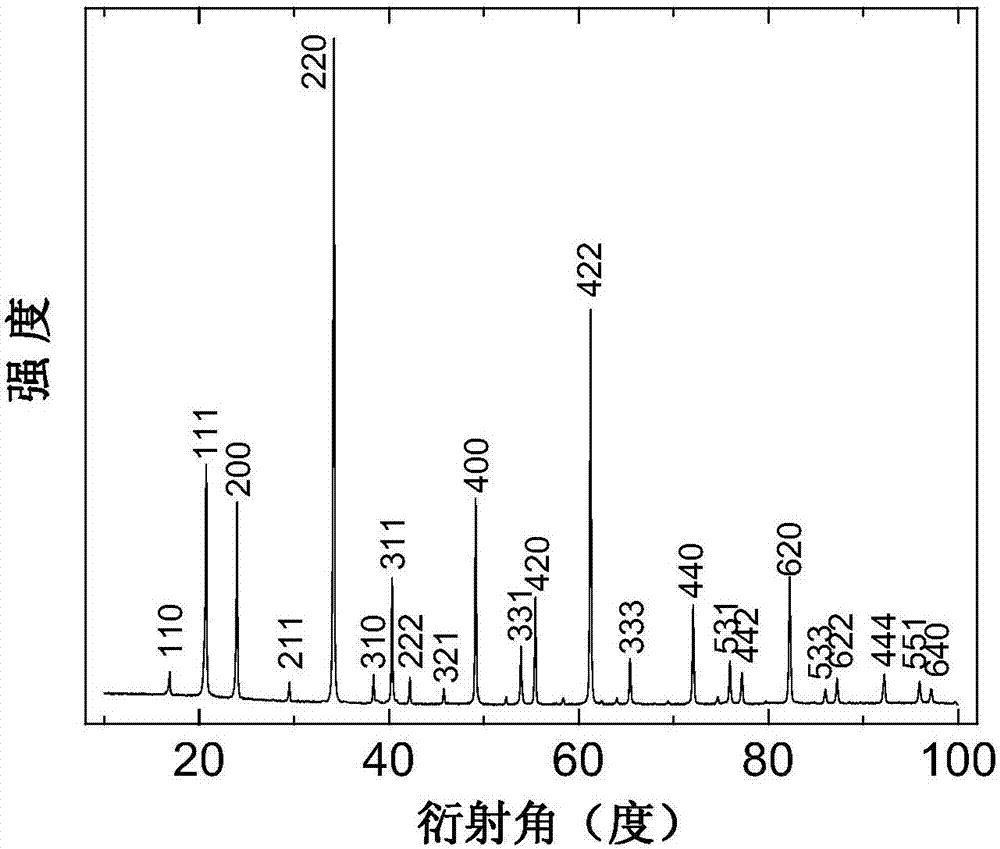
[0041] NaOH, Fe with a purity higher than 99.9% 2 O 3 , CuO, Os and KClO 4 They were mixed in a molar ratio of 1:1:3:2:1.5, and ground in an argon-filled glove box for 30 minutes to obtain a mixture with a particle size of 200 mesh. The mixture was filled and sealed in gold capsules with a wall thickness of 0.1 mm. The gold capsules are placed in a six-sided top press, and under the conditions of a pressure of 8 GPa and a temperature of 1100° C., the raw materials in the gold capsules are reacted for 30 minutes to obtain a reaction product. After the temperature dropped uniformly to room temperature within 2 hours, the reaction product was taken out of the gold capsule and ground again. NaCu was obtained by washing with deionized water 3 Fe 2 Os 2 O 12 .
[0042] In this example, KClO 4 As an oxygen source, it is used to provide O atoms. Deionized water was used to remove KCl entrapped in the reaction product.
Embodiment 2
[0044] NaOH, Fe with a purity higher than 99.9% 2 O 3 , CuO, Os, and NaClO 4 They were mixed in a molar ratio of 1:1:3:2:1.5, and ground in an argon-filled glove box for 30 minutes to obtain a mixture with a particle size of 200 mesh. The mixture was filled and sealed in platinum capsules with a wall thickness of 0.1 mm. The platinum capsules are placed in a six-sided top press, and the raw materials in the gold capsules are reacted for 30 minutes under the conditions of a pressure of 8 GPa and a temperature of 1100° C. to obtain a reaction product. The heating power was directly cut off, and after the temperature dropped to room temperature within 15 seconds, the reaction product was taken out from the platinum capsule and ground again. NaCu was obtained by washing with deionized water 3 Fe 2 Os 2 O 12 .
[0045] In this example, NaClO 4 As an oxygen source, it is used to provide O atoms. Deionized water was used to remove NaCl contained in the reaction product.
Embodiment 3
[0047] NaOH, Fe with a purity higher than 99.9% 2 O 3 , CuO, Os and KClO 4 The mixture was mixed in a molar ratio of 1:1:3:2:1.375, and ground in an argon-filled glove box for 60 minutes to obtain a mixture with a particle size of 500 mesh. The mixture was filled and sealed in gold capsules with a wall thickness of 0.1 mm. The gold capsules are placed in a six-sided top press, and the raw materials in the gold capsules are reacted for 10 minutes under the conditions of a pressure of 6 GPa and a temperature of 1200° C. to obtain a reaction product. After the temperature dropped uniformly to room temperature within 10 hours, the reaction product was taken out of the gold capsule and ground again. NaCu was obtained by washing with deionized water 3 Fe 2 Os 2 O 12 .
[0048] In this example, KClO 4 As an oxygen source, it is used to provide O atoms. Deionized water was used to remove KCl entrapped in the reaction product.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Curie temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com