Synthesis method of 3-bromo-5-methylpyridine
A kind of methylpyridine and synthetic method technology, applied in directions such as organic chemistry, can solve the problems of low yield, long process route, etc., and achieve the effects of high yield, short process route and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach 1
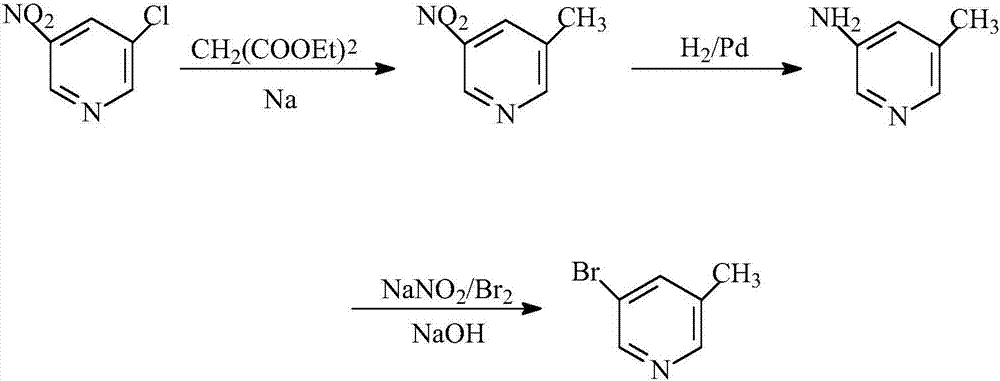
[0024] Diethyl malonate reacts with sodium to generate a salt, then adds dropwise a toluene solution of 3-nitro-5-chloropyridine for condensation reaction, and then decarboxylates under acidic conditions to obtain 3-nitro-5-methylpyridine; The molar ratio of diethyl diacid, alkali metal, and 3-nitro-5-chloropyridine is 5:1.1:1.
[0025] 3-nitro-5-methylpyridine was catalyzed by Pd / C, methanol was used as solvent, hydrogenated for reduction, suction filtered, and the filtrate was concentrated to obtain 3-amino-5-methylpyridine.
[0026] 3-Amino-5-picoline first forms a salt with an acid, cools to -10°C, adds liquid bromine dropwise, and then adds a sodium nitrite aqueous solution dropwise, after the dropwise addition, adjust the pH of the solution to be alkaline, then extract, dry, Concentration gave 3-bromo-5-methylpyridine.
Embodiment approach 2
[0028] Diethyl malonate reacts with sodium to generate a salt, then adds dropwise a toluene solution of 3-nitro-5-chloropyridine for condensation reaction, and then decarboxylates under acidic conditions to obtain 3-nitro-5-methylpyridine; The molar ratio of diethyl diacid, alkali metal, and 3-nitro-5-chloropyridine is 6:1.3:1;
[0029] 3-nitro-5-picoline is catalyzed by Pd / C, methanol is used as a solvent, hydrogenated for reduction, suction filtered, and the filtrate is concentrated to obtain 3-amino-5-picoline;
[0030] 3-Amino-5-methylpyridine forms a salt with an acid first, cools to 0°C, adds liquid bromine dropwise, and then adds a sodium nitrite aqueous solution dropwise, after the dropwise addition, adjust the pH of the solution to be alkaline, then extract, dry, and concentrate , in 3-bromo-5-methylpyridine.
Embodiment approach 3
[0032] Diethyl malonate reacts with potassium to generate a salt, then adds dropwise a toluene solution of 3-nitro-5-chloropyridine for condensation reaction, and then decarboxylates under acidic conditions to obtain 3-nitro-5-methylpyridine; The molar ratio of diethyl diacid, alkali metal, and 3-nitro-5-chloropyridine is 5.4:1.2:1;
[0033] 3-nitro-5-methylpyridine is catalyzed by Pd / C, methanol is used as a solvent, hydrogenated for reduction, suction filtered, and the filtrate is concentrated to obtain 3-amino-5-methylpyridine;
[0034] 3-Amino-5-picoline first forms a salt with an acid, cools to -4°C, adds liquid bromine dropwise, and then adds a sodium nitrite aqueous solution dropwise, after the dropwise addition, adjust the pH of the solution to be alkaline, then extract, dry, Concentration gave 3-bromo-5-methylpyridine.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com