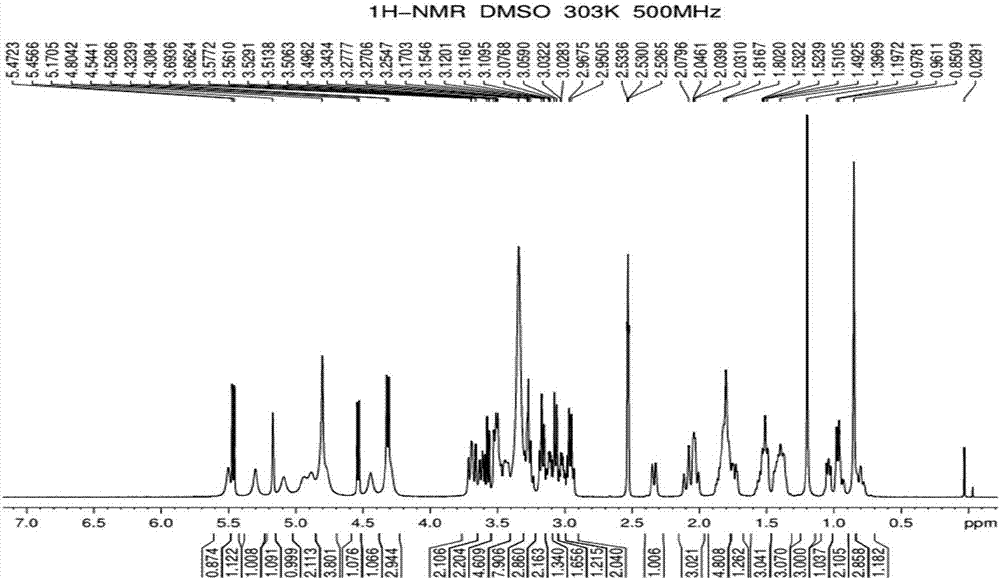
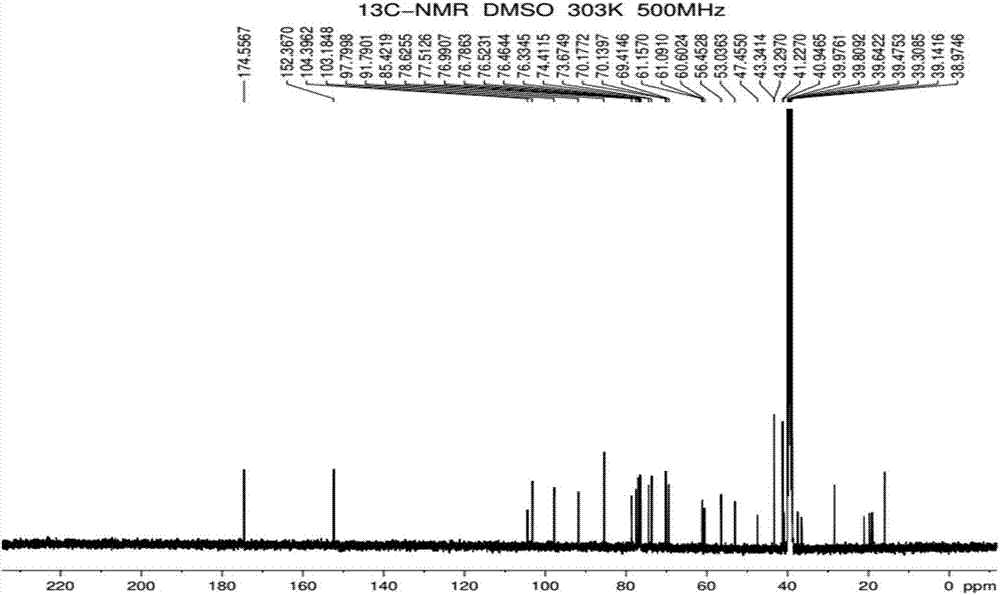
Preparation method of rebaudioside-KA
A glucose and amino acid technology, applied in biochemical equipment and methods, transferases, enzymes, etc., can solve the problems of uncontrollable reaction process, low substrate selectivity, and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1 Glycosyltransferase recombinant expression in Escherichia coli
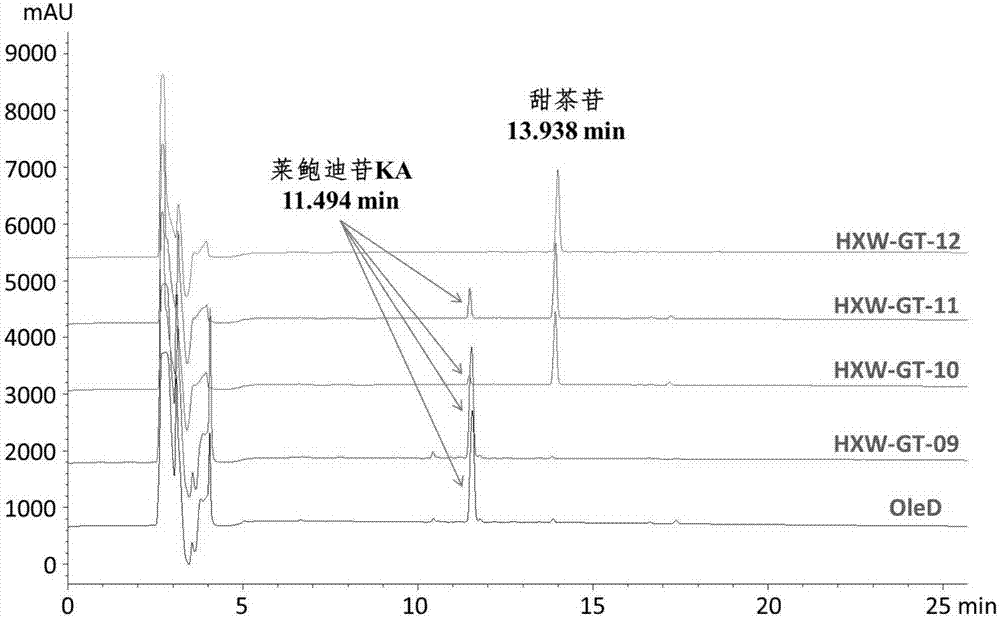
[0031] Design OleD homologous proteins: HXSW-GT-09 and HXSW-GT-10 were obtained by selecting multiple key amino acid residue mutations based on OleD structure and catalytic mechanism; HXSW-GT-11 and HXSW-GT-12 were obtained based on multiple sequences Alignment and OleD structure were obtained by mutation of key amino acid residues and motif recombination of UDP-glucose or substrate binding related parts. HXSW-GT-09 (amino acid sequence shown in SEQ ID NO: 2, nucleotide sequence shown in SEQ ID NO: 7), HXSW-GT-10 (amino acid sequence shown in SEQ ID NO: 3, nucleotide acid sequence as shown in SEQ ID NO:8), HXSW-GT-11 (amino acid sequence as shown in SEQ ID NO:4, nucleotide sequence as shown in SEQ ID NO:9), HXSW-GT-12 (amino acid The sequence is shown in SEQ ID NO:5, and the nucleotide sequence is shown in SEQ ID NO:10).
[0032] The genes of the glycosyltransferase OleD (the amino acid sequ...
Embodiment 2
[0036] Example 2 Recombinant expression of glycosyltransferase in yeast
[0037] The glycosyltransferase OleD and its homologous protein genes were respectively constructed into the Pichia pastoris protein expression vector pPIC9K, and then the recombinant expression plasmid was transformed into Pichia pastoris GS115 by electric shock, and positive transformants were screened according to the Pichia pastoris operation manual of Invitrogen Company (G418 resistance screening). Select a single clone and inoculate it in LB medium, culture it overnight at 30°C to obtain a bacterial solution, connect it to BMGY medium at a 1% inoculum size (v / v), culture it at 30°C and 250 rpm for 48 hours, collect the bacteria by centrifugation, then resuspend the bacteria and mix Transplanted into BMMY, the expression was induced by methanol at 30°C and 250rpm for 5 days. After the induced expression was completed, SDS-PAGE was used to analyze the expression of the target protein in the medium su...
Embodiment 3
[0038] Example 3 Recombinant expression of glycosyltransferase in Bacillus subtilis
[0039] Glycosyltransferase OleD and its homologous protein genes were respectively constructed into Bacillus subtilis secretion expression vector pHT43, the recombinant expression plasmid was transformed into Bacillus subtilis WB800 by chemical method, and positive clones were screened on LB solid medium containing ampicillin. Pick positive colonies and inoculate them into LB liquid medium containing ampicillin, and cultivate to OD at 37°C 600 =0.5, add IPTG to induce overnight, centrifuge to obtain the supernatant of the culture medium, and analyze the expression of the target protein in the supernatant by SDS-PAGE. The results showed that the secreted expression levels of glycosyltransferases OleD, HXSW-GT-09, HXSW-GT-10, HXSW-GT-11 and HXSW-GT-12 in Bacillus subtilis WB800 were higher than those in yeast, respectively 0.33μg / ml, 0.31μg / ml, 0.27μg / ml, 0.25μg / ml, 0.28μg / ml, verified the fea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com