Acetoin synthesis method
A synthetic method and technology of acetoin, applied in the field of acetoin synthesis, can solve problems such as toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
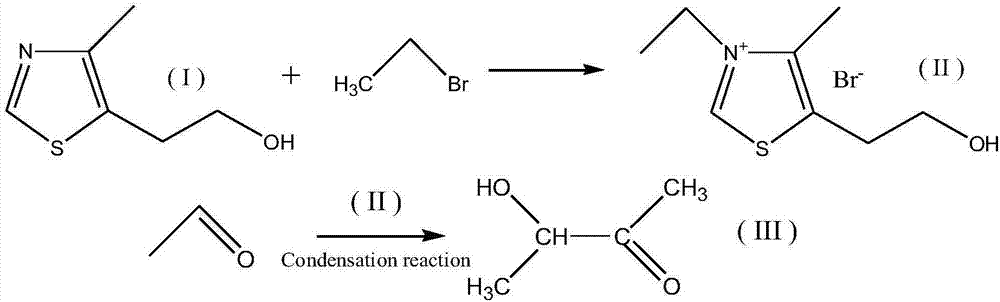
[0019] The invention provides a kind of synthetic method of acetoin, comprising the following steps:
[0020] Step 1, preparing the catalyst: after equimolar mixing of thiothiazole (I) and ethyl bromide, acetoin is used as a solvent, and the temperature is 150° C. to 160° C. in an oil bath for reflux reaction for 28 to 32 hours. After the reaction, Stir and cool down to room temperature, and recover the solvent by rotary evaporation. After standing still, suction filtration and washing, the precipitated white precipitate is recrystallized by acetoin and dried to obtain the catalyst 3-ethyl-4-methyl-5-hydroxyethyl Thiazolium bromide (II); the
[0021] Step 2, acetaldehyde condensation synthesis of acetoin: add catalyst 3-ethyl-4-methyl-5-hydroxyethylthiazole bromide (II) to the acetaldehyde solution, the molar ratio of acetaldehyde to catalyst is 500~ 600:1, and use sodium bicarbonate to adjust the pH value to 9-10, then heat it to 110-130°C in the pressure reactor, and stir t...
Embodiment 1
[0023] 1) To a three-necked flask equipped with a reflux device, sequentially add equimolar thiothiazole and ethyl bromide, and use acetoin as a solvent, reflux for 28 hours under heating in an oil bath at 150°C, and continue stirring until the reaction solution drops to room temperature , the solvent was recovered by rotary evaporation, the remaining reaction liquid was left to stand for 8 hours, suction filtered, washed with acetoin to obtain a white solid, and after acetoin was recrystallized, dried in a vacuum oven at 50°C to obtain 3-ethyl-4- Methyl-5-hydroxyethylthiazolium bromide.
[0024] 2) Add acetaldehyde and 3-ethyl 4-methyl-5-hydroxyethylthiazole bromide with a molar ratio of 500:1 into a 2L pressure reactor, and adjust the pH value of the reaction solution to 9 with sodium bicarbonate , start stirring and heat up to 110°C, the reaction starts automatically, the pressure of the reactor gradually rises to 1.5MPa, and drops to 0Mpa after 3 hours, indicating that the...
Embodiment 2
[0027] 1) To a three-necked flask equipped with a reflux device, add equimolar thiothiazole and ethyl bromide sequentially, and use acetoin as a solvent, reflux for 30 hours under heating in an oil bath at 155°C, and continue stirring until the reaction solution drops to room temperature , the solvent was recovered by rotary evaporation, the remaining reaction liquid was left to stand for 8 hours, suction filtered, washed with acetoin to obtain a white solid, and after acetoin was recrystallized, dried in a vacuum oven at 50°C to obtain 3-ethyl-4- Methyl-5-hydroxyethylthiazolium bromide.
[0028] 2) Add acetaldehyde and 3-ethyl 4-methyl-5-hydroxyethylthiazole bromide with a molar ratio of 550:1 into a 2L pressure reactor, and adjust the pH value of the reaction solution to 9.5 with sodium bicarbonate , start stirring and raise the temperature to 120°C, the reaction starts automatically, the pressure of the reactor gradually rises to 1.5MPa, and drops to 0Mpa after 3 hours, ind...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com