Marine biological polysaccharide-copper compound and its preparation method and application
A technology for marine biological polysaccharides and complexes, which is applied in the fields of botanical equipment and methods, chemicals for biological control, applications, etc., can solve the problems of difficult promotion, high cost, poor efficacy, etc. Good solubility and the effect of improving antibacterial activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
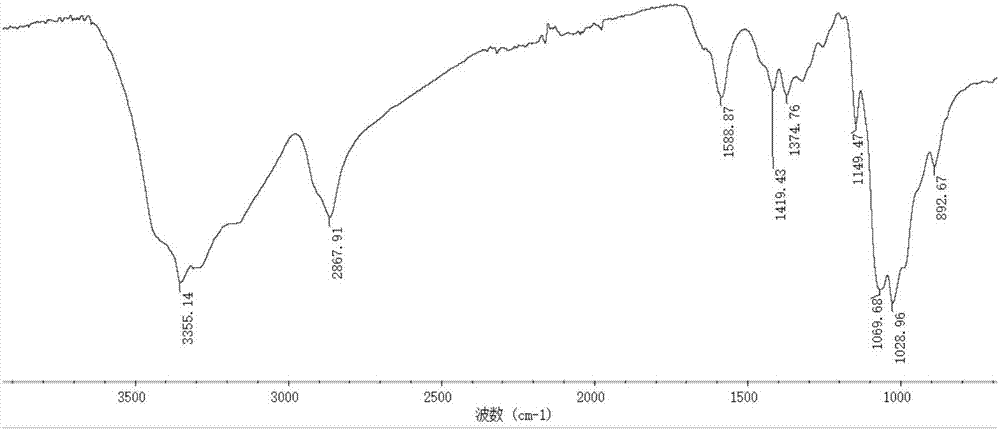
[0031] The preparation of embodiment 1 derivative 1
[0032] Add 4.7 grams of 2-aminopyridine and 5 grams of acetylacetone into 150 mL of absolute ethanol, and react under reflux for 12 hours. The reactant is rotary evaporated, washed with cold ethanol, and dried at 60 degrees Celsius to obtain the monoquaternary ammonium ligand.
[0033] Add 1.5 grams of chitosan with a molecular weight of 1.3 million to 60 mL of absolute ethanol and 75 mL of water, and add 1.5 mL of acetic acid dropwise under stirring; In the system, reflux for 15 hours; add 7.5 grams of chloroacetic acid to 20 mL of absolute ethanol, dropwise add to the reaction system, react at 60 ° C for 6 hours, cool to room temperature, add absolute ethanol to precipitate, precipitate with suction filtration, anhydrous Wash with ethanol and dry at 60°C to obtain light yellow powder, which is O-carboxymethyl chitosan Schiff base derivative.
[0034] Dissolve 1.0 g of O-carboxymethyl chitosan Schiff base derivative in 60...
Embodiment 2
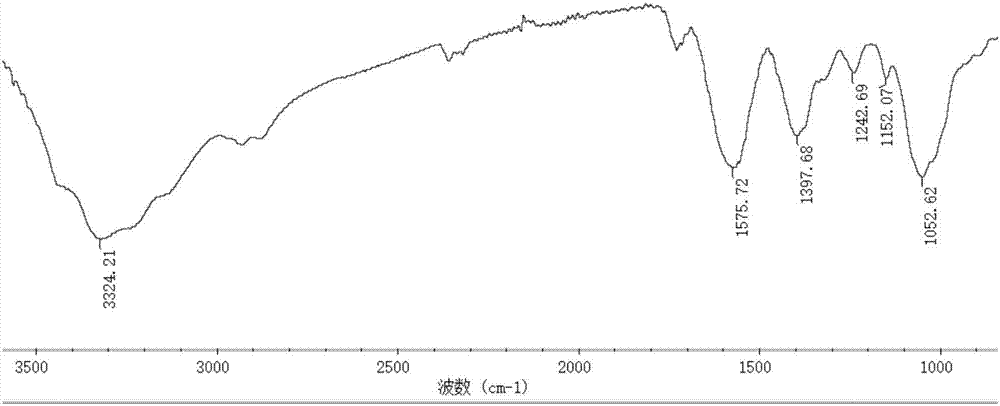
[0037] The preparation of embodiment 2 derivative 2
[0038] 5.4 grams of 2-amino-5-picoline and 5 grams of acetylacetone were added to 150 mL of absolute ethanol, refluxed for 12 hours, the reactant was rotary evaporated, washed with cold ethanol, and dried at 60 degrees Celsius to obtain the monoquaternary amine compound body.
[0039] Add 1.5 grams of chitosan with a molecular weight of 1.3 million to 60 mL of absolute ethanol and 75 mL of water, and add 1.5 mL of acetic acid dropwise under stirring; In the system, reflux for 15 hours; add 7.5 grams of chloroacetic acid to 20 mL of absolute ethanol, dropwise add to the reaction system, react at 60 ° C for 6 hours, cool to room temperature, add absolute ethanol to precipitate, precipitate with suction filtration, anhydrous Wash with ethanol and dry at 60°C to obtain light yellow powder, which is O-carboxymethyl chitosan Schiff base derivative.
[0040] Dissolve 1.0 g of O-carboxymethyl chitosan Schiff base derivative in 60...
Embodiment 3
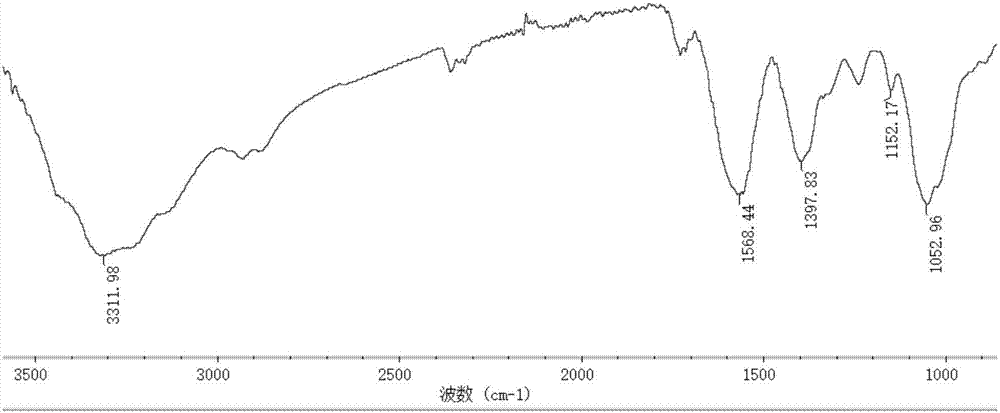
[0041] The preparation of embodiment 3 derivative 3
[0042] Add 6.4 grams of 2-amino-5-chloropyridine and 5 grams of acetylacetone to 150 mL of absolute ethanol, reflux for 12 hours, spin evaporate the reactants, wash with cold ethanol, and dry at 60 degrees Celsius to obtain the monoquaternary ammonium ligand .
[0043] Add 1.5 grams of chitosan with a molecular weight of 1.3 million to 60 mL of absolute ethanol and 75 mL of water, and add 1.5 mL of acetic acid dropwise under stirring; In the system, reflux for 15 hours; add 7.5 grams of chloroacetic acid to 20 mL of absolute ethanol, dropwise add to the reaction system, react at 60 ° C for 6 hours, cool to room temperature, add absolute ethanol to precipitate, precipitate with suction filtration, anhydrous Wash with ethanol and dry at 60°C to obtain light yellow powder, which is O-carboxymethyl chitosan Schiff base derivative.
[0044] Dissolve 1.0 g of O-carboxymethyl chitosan Schiff base derivative in 60 mL of water, 1....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com