Preparation of a palladium hydrogen nanoparticle and its application in electrocatalytic oxidation of formic acid
A nanoparticle and reaction vessel technology, applied in the field of palladium hydrogen nanocatalytic materials, can solve the problems of unadjustable Pd and H ratio, limited application, insufficient stability of PdH, etc., achieve superior catalytic activity, improve catalytic activity, and reduce oxidation overpotential Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
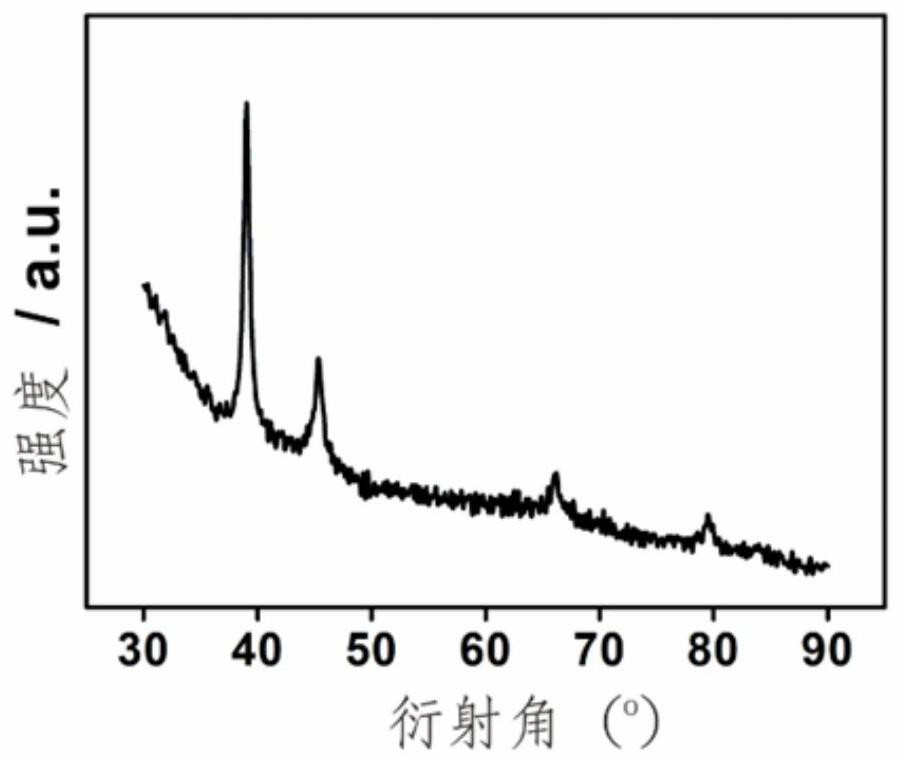
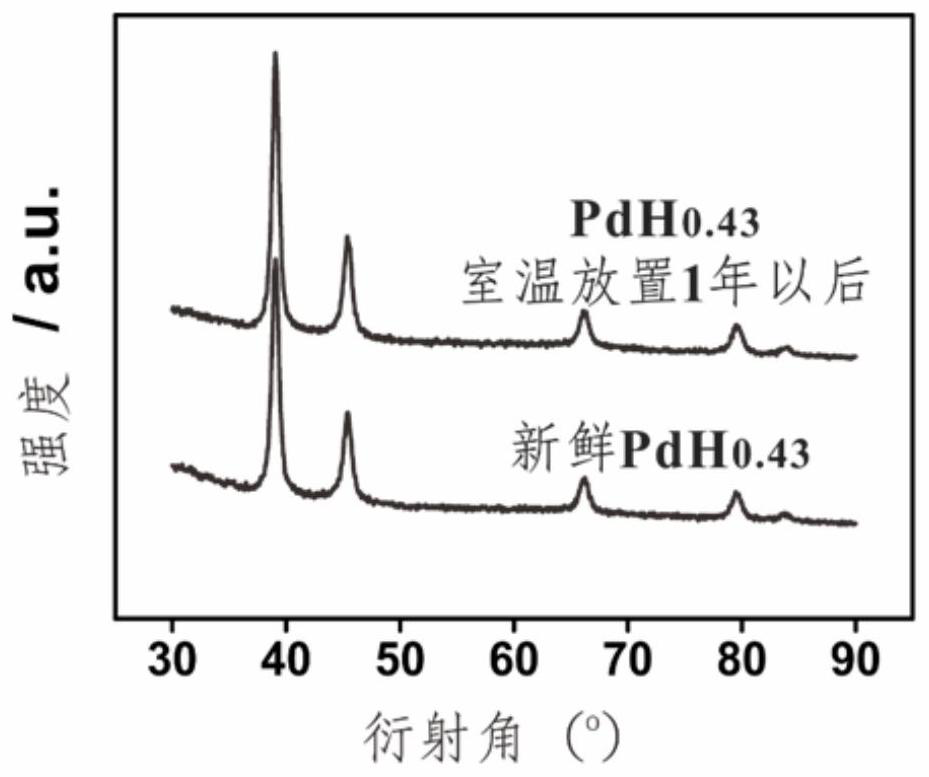
[0024] In a 25 mL reactor, 5 mg of commercial Pd black and 10 mL of n-butylamine were mixed evenly, and then the reactor containing the reaction solution was placed in an oven, and the temperature was raised from room temperature to 150 °C and kept at a constant temperature for 5.0 h, and then cooled down to room temperature naturally. Finally, all the products were collected by centrifugation, and the samples were washed with ethanol several times to remove the adhesive on the surface. Its phase analysis is a β-phase palladium hydrogen compound, which belongs to the face-centered cubic structure, and the X-ray powder diffraction is as follows: figure 1 , 3 ~5 shown.
Embodiment 2
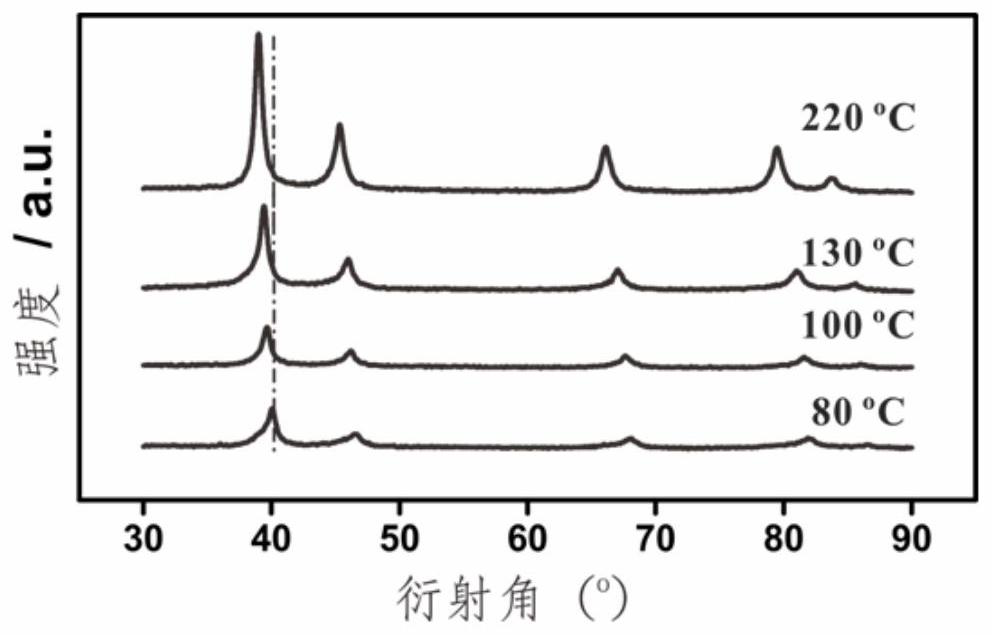
[0026] In a 25 mL reactor, 5 mg of commercial Pd black and 10 mL of n-butylamine were mixed evenly, and then the reactor containing the reaction solution was placed in an oven, and the temperature was raised from room temperature to 220 °C and kept at a constant temperature for 5.0 h, and then cooled down to room temperature naturally. Finally, all the products were collected by centrifugation, and the samples were washed with ethanol several times to remove the adhesive on the surface. Its phase analysis is identical with embodiment 1, and corresponding X-ray powder diffraction is as figure 2 shown.
Embodiment 3
[0028] In a 25 mL reactor, 5 mg of commercial Pd black and 10 mL of n-butylamine were mixed evenly, and then the reactor containing the reaction solution was placed in an oven, and the temperature was raised from room temperature to 130 °C and kept at a constant temperature for 5.0 h, and then cooled down to room temperature naturally. Finally, all the products were collected by centrifugation, and the samples were washed with ethanol several times to remove the adhesive on the surface. Its phase analysis is the same as in Example 1, and the ratio of H and Pd in its palladium hydride sample is 0.33. The corresponding X-ray powder diffraction figure 2 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com