Human placenta chorion mesenchymal stem cell culture system
A technology of stem cells and culture system, applied in the field of biomedicine, can solve the problems of high chance of virus infection, unacceptable, decreased differentiation ability and cell number, etc., to achieve the effect of benefiting clinical application and improving utilization rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
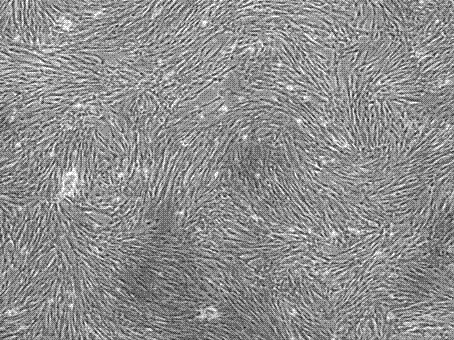
[0041] Example 1: Human placental chorionic mesenchymal stem cell culture
[0042]After the human placental chorionic tissue was treated, the primary culture was performed. After 7 days of static culture, the medium was completely replaced. The tissue blocks, culture medium and washing fluid in the primary culture flask were re-inoculated after centrifugation, and then cultured again. After 7 days of static culture, the full amount was replaced. culture medium; re-inoculate the tissue pieces, culture fluid and washing fluid in the culture flask after centrifugation, carry out the third culture, and replace the culture medium in full after 7 days of static culture; when the confluence of the three cultured cells reaches 80-90%, use Trypsin digestion and passage to obtain human placental chorionic mesenchymal stem cells. The number of cells obtained from the primary culture, the re-culture and the third culture and the harvest time are shown in Table 1.
[0043] cultur...
Embodiment 4
[0049] Example 4: Passaging, cryopreservation and recovery of human placental chorionic mesenchymal stem cells
[0050] When the cells in the flask reach 80-90% confluence, remove the medium, rinse the flask twice with normal saline, add 3ml of trypsin, place it in a 37°C incubator to digest for about 3-5 minutes, and add the medium 15ml, stop trypsin digestion, and take 100ul for counting cell viability and cell number. After centrifugation (1500r / min, 5min), the cells were subcultured at a ratio of 1:2 and placed in a saturated humidity, 37°C, and a volume fraction of 5% CO. 2 In the incubator, generally 80-90% confluent state can be reached in 2-3 days, and it needs to be passaged again. When the passage that needs to be cryopreserved is reached, according to the passage of digestion, according to the number of cells, add culture medium and cell cryopreservation solution to make the cell concentration 1 × 10 6 / ml, the cell cryopreservation solution is 10%. After mixing,...
Embodiment 5
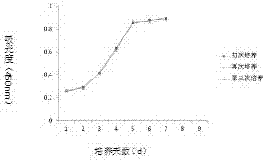
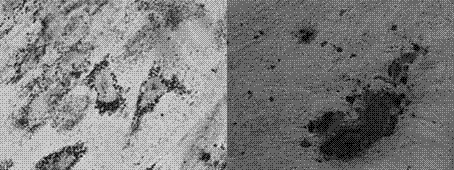
[0051] Example 5: Identification of biological characteristics of human placental chorionic mesenchymal stem cells and comparison of three cultured cells
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com