Anti AFP (alpha-fetoprotein) CAR-T (chimeric antigen receptor T) cell, preparation method thereof and application of cell
A technology of cells and lymphocytes, applied in the field of gene-modified cells and tumor treatment, which can solve the problems of high cost of immune rejection, shortage of donors, surgical injuries, etc., and achieve the effect of high-efficiency tumor-killing activity and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Preparation of Anti AFP CAR-T cells of the present invention
[0046] 1. Construction of lentiviral expression vector
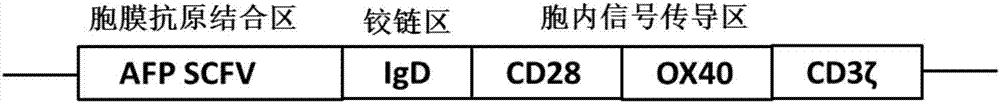
[0047] Through conventional genetic engineering methods, the SCFV-IgD-CD28-OX40-CD3ζ fusion protein gene sequence was synthesized, and the SCFV-IgD-CD28-OX40-CD3ζ fusion protein gene sequence was cloned into a lentiviral expression vector to obtain SCFV-IgD-CD28 –OX40–CD3ζ lentiviral expression vector pGreen puro-CAR. Wherein, each sequence of the SCFV-IgD-CD28-OX40-CD3ζ fusion protein is as described above.
[0048] 2. Lentiviral packaging
[0049] 1) Culture 293T cells in 1640 medium with a mass fraction of 10 wt% fetal bovine serum (fetal bovine serum, FBS for short);
[0050] 2) 293T cells were treated with 3x 10 5 / cm 2 Transfer the density to a culture dish with a diameter of 15cm and cultivate it for 20h to ensure that the cell confluence is 80-90% during transfection;
[0051] Replace the medium with serum-free 1640 medium and set aside; ...
Embodiment 2
[0065] The in vitro anti-tumor effect of the Anti AFP CAR-T cells prepared in Example 1 was evaluated, including the following steps:
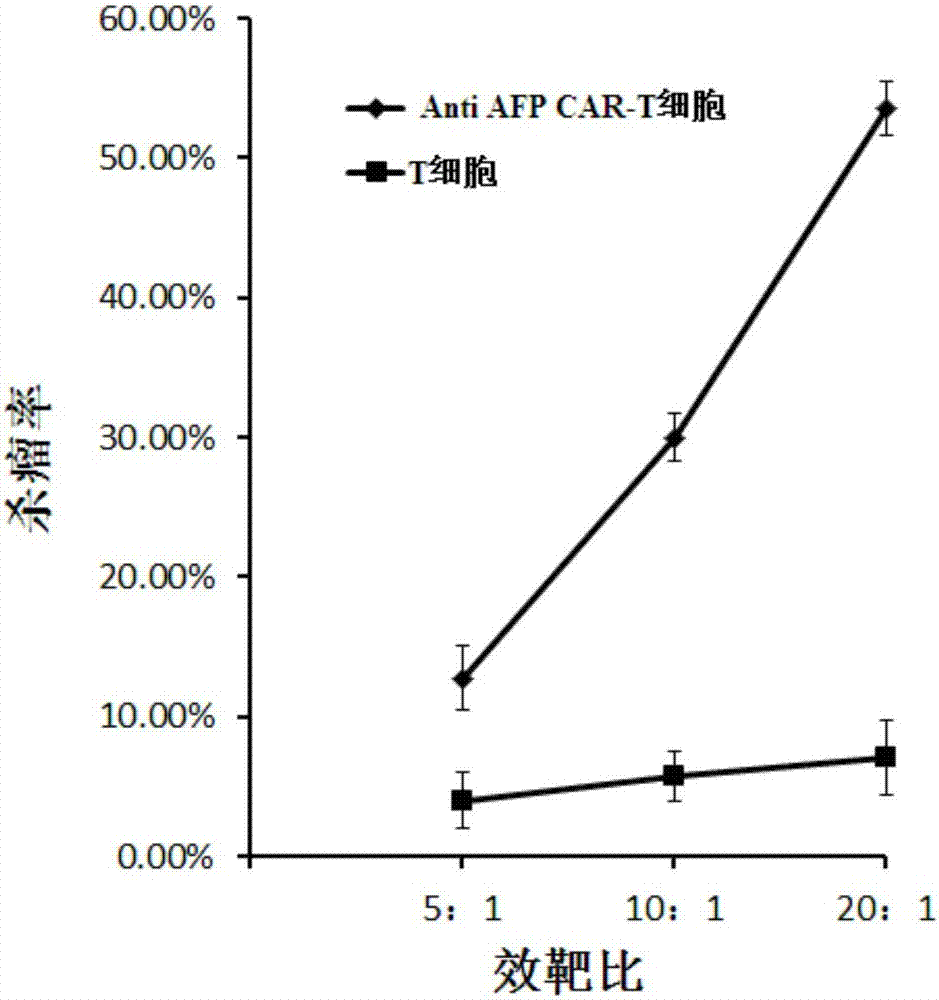
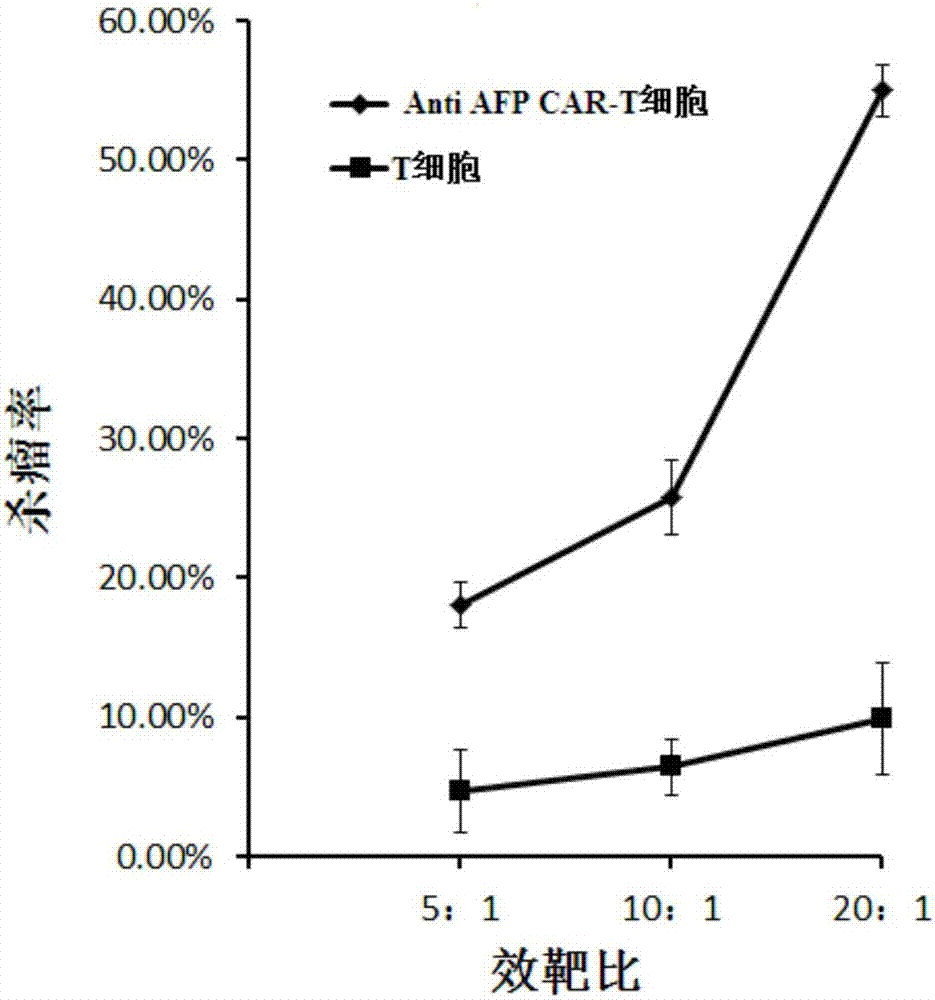
[0066] With the liver cancer cell lines HepG-2 and Hep3B stably expressing AFP as target cells, T cells infected with the lentiviral vector pGreen puro-CAR (that is, the Anti AFP CAR-T cells prepared in Example 1) and uninfected T cells were respectively used. Make effector cells from cells, and target cells at a density of 1x 10 5 cells / ml inoculated in 96-well plate, 100 μl per well, added effector cells to target cells according to 5:1, 10:1, 20:1 effect-to-target ratio, placed in 5% CO 2 , cultured in a 37°C incubator for 4 hours, using WST-1 to detect cell viability, and calculate the killing efficiency.
[0067] figure 2 It is a schematic diagram of the killing effect of Anti AFP CAR-T cells and uninfected T cells on the target cell HepG-2 (liver cancer) according to the embodiment of the present invention; image 3 It is a schematic...
Embodiment 3
[0070] The anti-tumor effect of the Anti AFP CAR-T cells prepared in Example 1 was evaluated in vivo, including the following steps:
[0071] Take 15 6-week-old female nude mice and inject 5x 10 subcutaneously in the right armpit 6 HepG-2 (liver cancer) cells, when the tumor grows to 60mm 3 Size, tumor model was randomly divided into 3 groups: control group, Anti AFP CAR-T cell group, and T cell group; control group was injected with saline 200ul / time, twice a week; Anti AFP CAR-T cell group was injected with tail vein Anti AFP CAR-T cells 1×10 7 T cells / time, 2 times a week; T cells in the T cell group were injected into the tail vein 1×10 7 Each time, twice a week; the survival status of the mice within 100 days was counted, and the survival rate curve was made. Figure 4 It is a schematic diagram of the survival period of the mice treated with liver cancer (HepG-2) xenografted tumor model mice by the Anti AFP CAR-T cells of the embodiment of the present invention, the co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com