Cadmium ion adsorbent and preparation method and application thereof
A technology of cadmium ion and adsorbent, which is applied in the field of cadmium ion adsorbent and its preparation, can solve the problems of low adsorption capacity of activated carbon, difficult recycling and poor recycling performance, etc., and achieve fast adsorption rate, low cost and stable performance Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] A kind of preparation method of cadmium ion adsorbent, concrete steps are as follows:
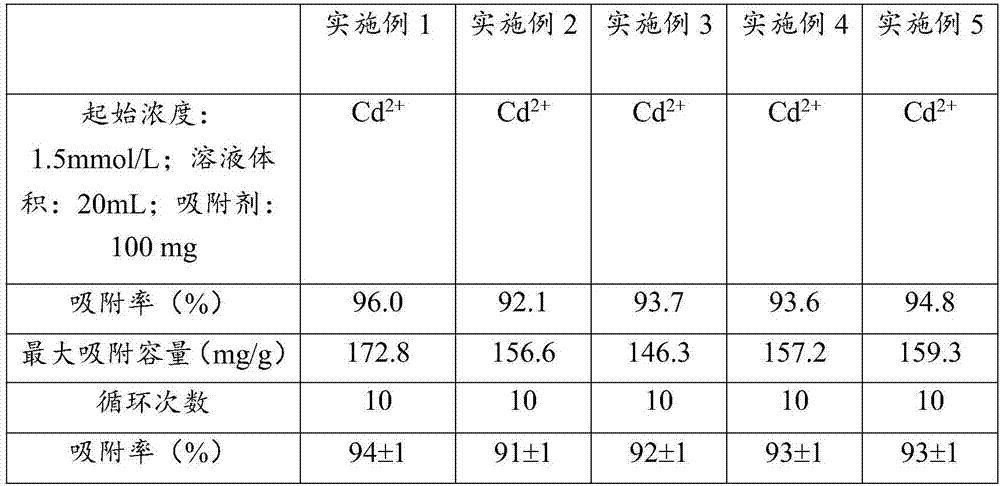
[0030] (1) Weigh 1.0g of sodium alginate and 1.0g of disodium ethylenediaminetetraacetate and dissolve them in 50mL of water, and add 0.35g of 1-ethyl-(3-dimethylaminopropyl)carbodiethylene Amine hydrochloride and 0.25g N-hydroxysuccinimide were activated for 1h, then 10mL of ethylenediamine was added, and reacted at 25°C for 4h to obtain solution I;
[0031] (2) under stirring condition, solution 1 is dripped in the calcium nitrate solution of 0.2mol / L dropwise, continue to stir 30min after dripping, obtain solid-liquid mixture;
[0032] (3) The solid after filtering the solid-liquid mixture is washed with distilled water, and then subjected to vacuum freeze-drying to obtain the cadmium ion adsorbent. The scanning electron microscope image of the cadmium ion adsorbent is as follows: figure 1 shown.
Embodiment 2
[0034] A kind of preparation method of cadmium ion adsorbent, concrete steps are as follows:
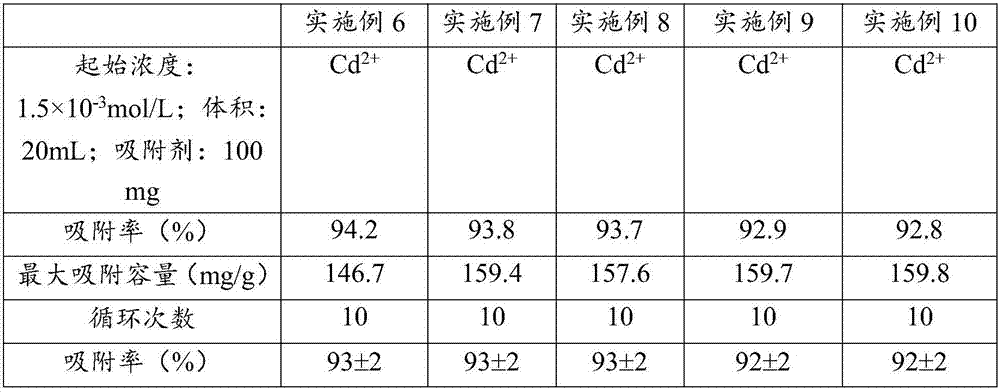
[0035] (1) Weigh 1.0g of potassium alginate and 1.0g of disodium ethylenediaminetetraacetate and dissolve it in 50mL of water, and add 0.35g of 1-ethyl-(3-dimethylaminopropyl)carbodiethylene Amine hydrochloride and 0.25g N-hydroxysuccinimide were activated for 1h, then 10mL of ethylenediamine was added, and reacted at 25°C for 4h to obtain solution I;
[0036] (2) under stirring condition, solution 1 is dripped in the calcium nitrate solution of 0.2mol / L dropwise, continue to stir 30min after dripping, obtain solid-liquid mixture;
[0037] (3) The solid after filtering the solid-liquid mixture is washed with distilled water, and then subjected to vacuum freeze-drying to obtain the cadmium ion adsorbent.
[0038] The difference between Example 2 and Example 1 is that sodium alginate is replaced by potassium alginate.
Embodiment 3
[0040] A kind of preparation method of cadmium ion adsorbent, concrete steps are as follows:
[0041] (1) Weigh 1.0g of alginic acid and 1.0g of disodium ethylenediaminetetraacetate and dissolve it in 50mL of water, and add 0.35g of 1-ethyl-(3-dimethylaminopropyl) carbodiimide under stirring condition Hydrochloride and 0.25g N-hydroxysuccinimide were activated for 1h, then 10mL of ethylenediamine was added, and reacted at 25°C for 4h to obtain solution I;
[0042] (2) under stirring condition, solution 1 is dripped in the calcium nitrate solution of 0.2mol / L dropwise, continue to stir 30min after dripping, obtain solid-liquid mixture;
[0043] (3) The solid after filtering the solid-liquid mixture is washed with distilled water, and then subjected to vacuum freeze-drying to obtain the cadmium ion adsorbent.
[0044] The difference between Example 3 and Example 1 is that sodium alginate is replaced by alginic acid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com