Dronedarone hydrochloride osmotic pump type controlled release tablet
A dronedarone hydrochloride and osmotic pump technology, which is applied in the field of dronedarone osmotic pump controlled-release tablets, can solve the problems of large side effects, low blood drug concentration, and low bioavailability of patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
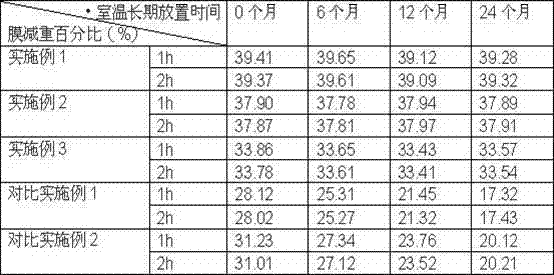
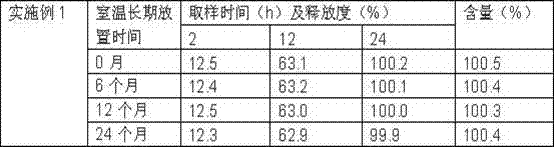
Embodiment 1
[0047] 1. Prescription
[0048] 1) Tablet core prescription, based on 1000 tablets:
[0049] Drug-containing layer:
[0050] Dronedarone Hydrochloride 200-500g
[0051] Lactose 320g
[0052] Cyclodextrin 80g
[0053] Microcrystalline Cellulose 120g
[0054] Polyethylene glycol 50g
[0055] Microcrystalline Cellulose 32g
[0057] Boost layer:
[0058] Hydroxypropyl Methyl Cellulose 130g
[0060] Lactose 100g
[0061] Cellulose acetate 60g
[0063] 2) Prescription of semi-permeable membrane coating solution
[0064] Cellulose acetate 74g
[0065] Polycarbonate 33g
[0066] Ethanol 1000ml
[0067] 3) Prescription of film coating solution
[0068] Gastric coating powder 10g
[0069] water 100ml
[0070] 2. Detailed preparation process
[0071] 1. Preparation process of dronedarone hydrochloride tablet core: The tablet core is a double-layer tablet, one layer is a drug-...
Embodiment 2
[0080] 1. Prescription
[0081] 1) Tablet core prescription, based on 1000 tablets:
[0082] Drug-containing layer:
[0083] Dronedarone Hydrochloride 200-500g
[0084] Lactose 320g
[0085] Cyclodextrin 80g
[0086] Microcrystalline Cellulose 120g
[0087] Polyethylene glycol 50g
[0088] Microcrystalline Cellulose 32g
[0089] Magnesium Stearate 2g
[0090] Boost layer:
[0091] Hydroxypropyl Methyl Cellulose 130g
[0092] Magnesium chloride 80g
[0093] Lactose 100g
[0094] Cellulose acetate 60g
[0095] Magnesium Stearate 1g
[0096] 2) Prescription of semi-permeable membrane coating solution
[0097] Cellulose acetate 74g
[0098] Polycarbonate 31g
[0099] Ethanol 1000ml
[0100] 3) Prescription of film coating solution
[0101] Gastric coating powder 10g
[0102] water 100ml
[0103] Two, detailed preparation process is the same as embodiment 1
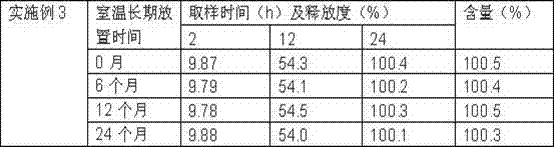
Embodiment 3 1
[0104] Embodiment 3 One, prescription
[0105] 1) Tablet core prescription, based on 1000 tablets:
[0106] Drug-containing layer:
[0107] Dronedarone Hydrochloride 200-500g
[0108] Lactose 320g
[0109] Cyclodextrin 80g
[0110] Microcrystalline Cellulose 120g
[0111] Polyethylene glycol 50g
[0112] Microcrystalline Cellulose 32g
[0113] Magnesium Stearate 2g
[0114] Boost layer:
[0115] Hydroxypropyl Methyl Cellulose 130g
[0116] Magnesium chloride 80g
[0117] Lactose 100g
[0118] Cellulose acetate 60g
[0119] Magnesium Stearate 1g
[0120] 2) Prescription of semi-permeable membrane coating solution
[0121] Cellulose acetate 74g
[0122] Polycarbonate 35g
[0123] Ethanol 1000ml
[0124] 3) Prescription of film coating solution
[0125] Gastric coating powder 10g
[0126] water 100ml
[0127] Two, detailed preparation process is the same as embodiment 1
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com