Tuberculosis vaccine candidate component and vaccine containing tuberculosis vaccine candidate component
A technology for tuberculosis and Mycobacterium tuberculosis, applied in the fields of immunology and molecular biology, can solve the problems of unclear effective antigenic components of genome and proteome, basic research needs to be strengthened, and no gene annotation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
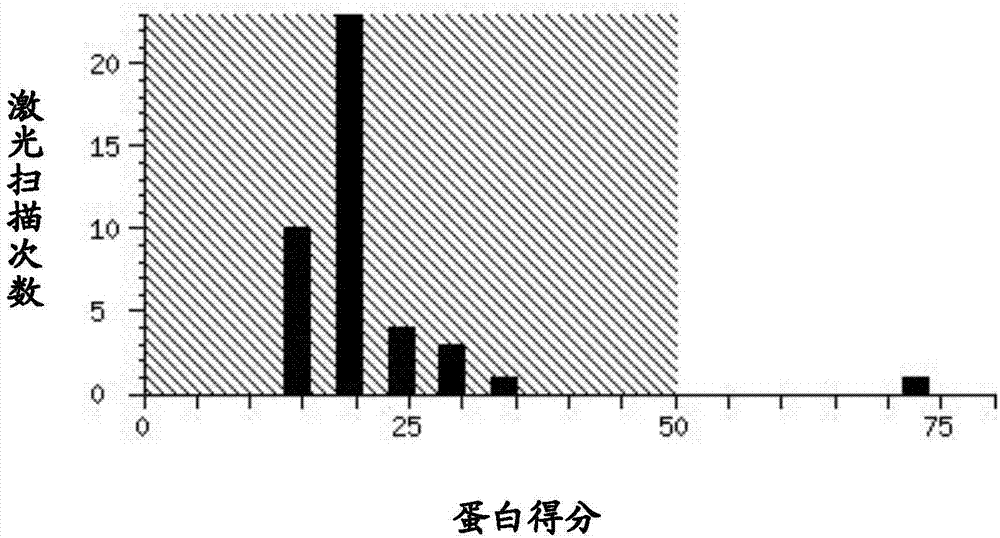
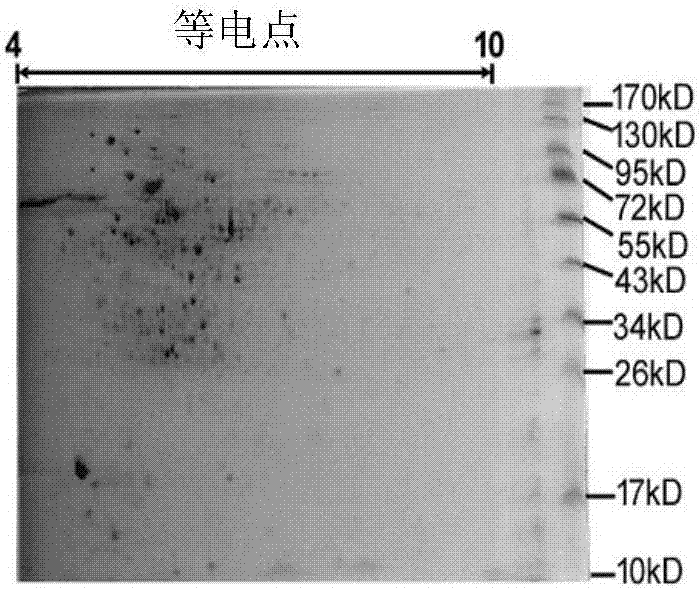
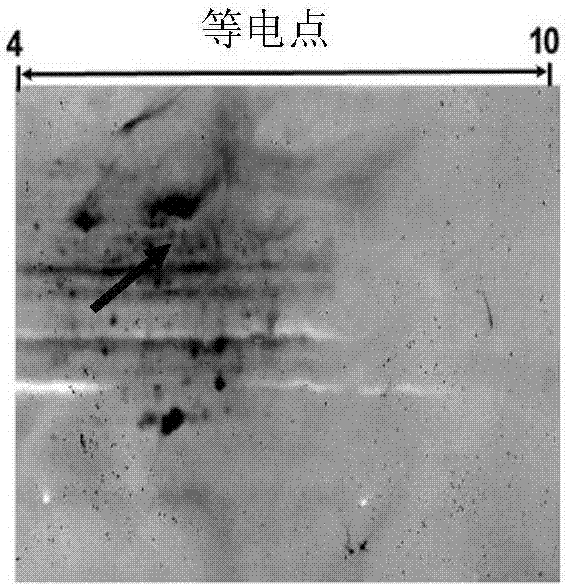
[0043] Example 1: Preparation and Identification of Protein MYVA_1927
[0044] The preparation of the protein MYVA_1927 (shown in SEQ ID NO:1) can be obtained by obtaining its coding base sequence (shown in SEQ ID NO:2), cloning it into an expression vector, and purifying it through protein expression. The coding base sequence can be prepared through synthetic chemical synthesis, and can also be cloned from Mycobacterium vaccae strain ATCC95051.
[0045] Specifically, the following methods can be used.
[0046] 1. Bacterial culture
[0047] Inoculate the frozen strain (ATCC 95051) into 200 ml of 7H9 liquid medium (10% OADC nutritional supplement, produced by BD Company, USA), cultivate overnight at 30°C, 100 rpm, subculture an appropriate amount according to 1:100 times, when the OD value When it reaches 0.8-1.0, place it on ice for 1.5 hours, centrifuge at 3000g for 10 minutes, and collect the strains.
[0048] A part of the collected strains was used for the genome extr...
Embodiment 2
[0095] Example 2: Protein MYVA_1927 specifically causes antibody production in tuberculosis patients
[0096] 1. Experimental materials
[0097] The strain collected in step 1 in Example 1.
[0098] Tuberculosis patient plasma: from 200 tuberculosis patients. Collected after informed consent of subjects. The determination of the patient meets any one of the following conditions: sputum smear or bacterial culture is positive; PCR amplification is positive (the target gene of PCR amplification is the conserved sequence fragment IS6110 of Mycobacterium tuberculosis, the copy number is 2x10 5 positive); chest X-ray or CT support is a tuberculosis patient; clinical ESAT-6 positive.
[0099] Healthy human plasma: from 50 healthy people. Collected after informed consent of subjects. The determination of healthy people meets all the following conditions: no clinical symptoms and chest X-ray confirmed healthy people, and ESAT-6 is negative.
[0100] 2. Experimental method
[0...
Embodiment 3
[0149] Embodiment 3: cellular immunity test
[0150] ELISPOT analysis enables the visualization of single-cell secreted products. These assays are exceptionally sensitive because it is captured directly around the secreting cell and before the surface is diluted, or captured by receptors on adjacent cells, or degraded. Autoimmune testing, organ transplantation, vaccine and drug development and testing, T cell function research, tumor research, infectious disease research and testing, virus research, etc. The specific principle is: cells are stimulated by antigens to produce cytokines, and the cytokine antigens are captured by specific monoclonal antibodies. After cell dissociation, the captured cytokines are bound to a biotin-labeled secondary antibody, followed by alkaline phosphatase-labeled avidin. After incubation with BCIP / NBT substrate, "purple" spots appeared on the PVDF plate, indicating that the cells had produced cytokines, and the results were obtained after ana...
PUM
| Property | Measurement | Unit |
|---|---|---|
| cover factor | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com