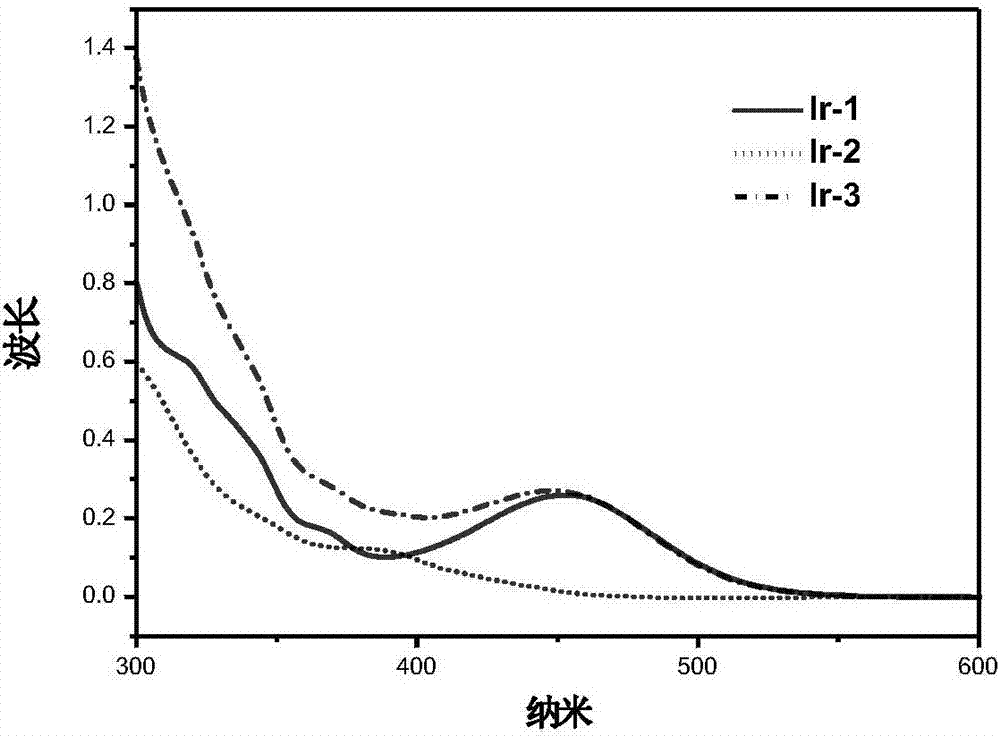
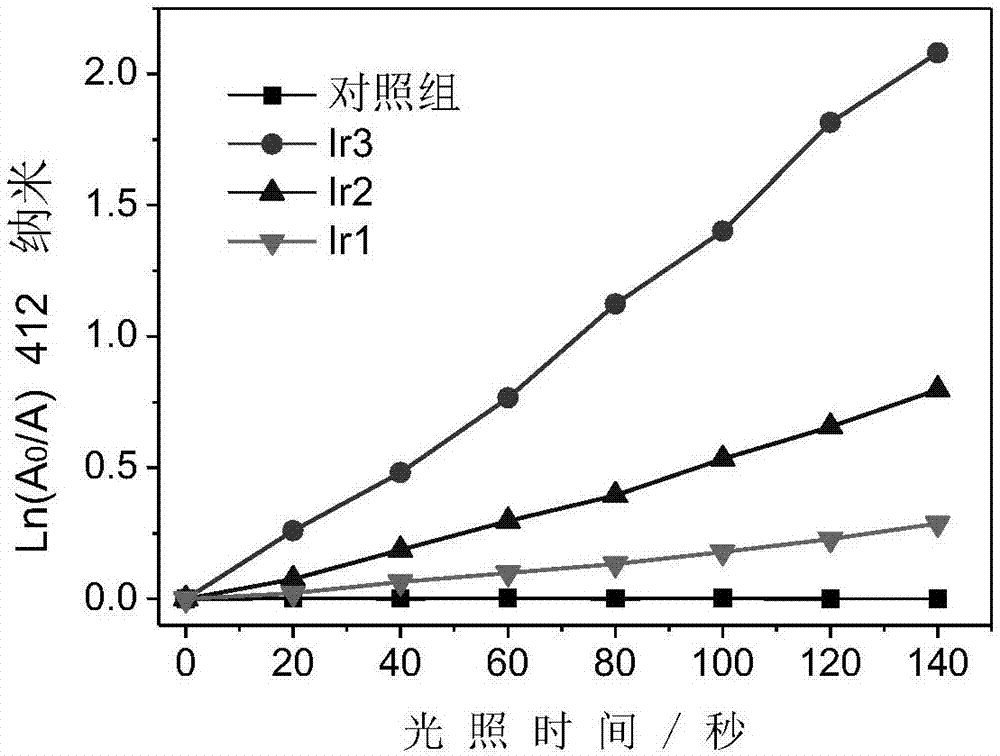
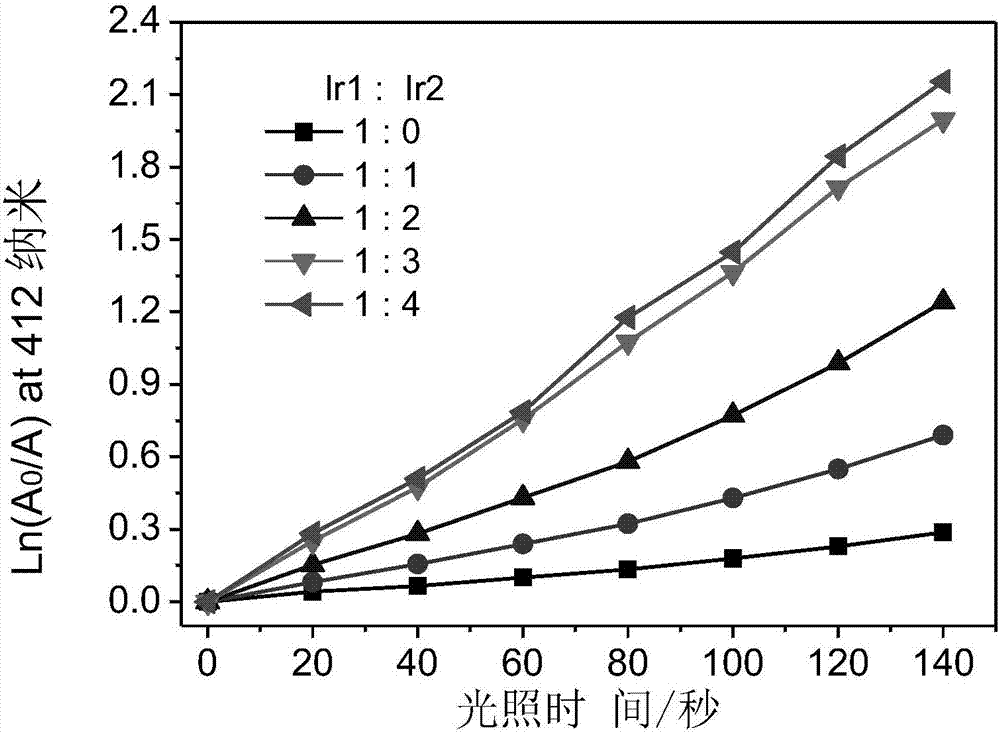
Iridium complex with phosphorescent ion pair structure as well as preparation method and application of iridium complex
A technology of iridium complex and ion pair, which is used in medical preparations containing active ingredients, indium organic compounds, platinum group organic compounds, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Preparation of cationic iridium complex Ir-1:
[0031] As shown in the figure above, as shown in Reaction i, weigh benzaldehyde (1mmol), 2-acetylpyridine (4mmol), sodium hydroxide (4.2mmol), ammonia water (3mmol) into the reaction flask, add ethanol, 25 ℃ Stir magnetically for 12 hours. After the reaction, the precipitate is filtered, and the recrystallized product obtained by recrystallization with ethanol is phenyl terpyridine, which can be directly put into the next reaction after drying. 1 H NMR (400MHz, CDCl 3 )δ8.76–8.71(m,4H),8.67(t,J=6.4Hz,2H),7.94–7.85(m,4H),7.49(dt,J=20.0,4.9Hz,3H),7.35(dt , J=10.8, 5.2Hz, 2H).
[0032] As shown in reaction ii, weigh phenyl terpyridine (1mmol) and IrCl 3 ·3H 2 O (1mmol) was mixed and put into a three-necked bottle, and the double-row tube was vacuumized-nitrogen gas-vacuumized, and the cycle was repeated three times, and finally the whole reaction system was protected by nitrogen gas. Ethylene glycol ether was injected i...
Embodiment 2
[0036] Preparation of anionic iridium complex Ir-2:
[0037] As shown in reaction ⅳ, weigh phenylpyridine (2.5mmol) and IrCl 3 ·3H 2O (1mmol) was mixed and put into a three-necked bottle, and the double-row tube was vacuumized-nitrogen gas-vacuumized, and the cycle was repeated three times, and finally the whole reaction system was protected by nitrogen gas. A mixture of ethylene glycol ether and water with a volume ratio of 3:1 was injected into the reaction system, the temperature was raised to 110° C., and the reaction was performed under magnetic stirring for 24 hours. After the reaction, cool the system to room temperature, filter the precipitate, and wash with ethanol and water. The obtained solid product is the phenylpyridine iridium dichloro bridge, which can be directly put into the next reaction after drying.
[0038] As shown in reaction v, weigh phenylpyridinium iridium dichloro bridge (1mmol) and tetrabutylcyanamide (2.3mmol) and mix them into the reaction flask...
Embodiment 3
[0041] Preparation of phosphorescent ion pair iridium complex Ir-3:
[0042] As shown in reaction ⅵ, weigh cationic iridium complex Ir-1 (1mmol) and anionic iridium complex Ir-2 (3mmol) and mix them into the reaction flask, inject acetone into the system, and control the temperature at 25°C. After stirring and reacting for 4 hours, a small amount of water was injected into the system and stirred for 1 minute, then suction filtered, and the solid product was recrystallized through acetone and ether to obtain a solid product which was the iridium complex Ir-3. 1 H NMR (400MHz, DMSO-d 6 )δ9.68(s,2H),9.61(s,2H), 9.30(s,1H),9.27–9.18(m,4H),8.59(d,J=8.5Hz,1H),8.44(d,J =7.6 Hz, 2H), 8.37(dt, J=14.5, 7.4Hz, 5H), 8.05(d, J=8.9Hz, 1H), 7.99(d, J=5.1Hz, 2H), 7.95(d, J =5.3Hz, 2H), 7.84(t, J=7.7Hz, 2H), 7.76(dd, J=12.8, 7.9Hz, 2H), 7.59(dt, J=12.6, 7.0Hz, 5H), 7.40(t ,J=7.6Hz, 1H),4.58(t,J=7.0Hz,2H),1.94–1.74(m,2H),1.50–1.17(m,6H), 0.82(t,J=7.1Hz,3H) .
[0043]
[0044] Same as ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com