Dehydroabietic-acid-based dipeptide surfactant and performance thereof
A surfactant, abietic acid-based technology, applied in the field of synthesis and application of natural product surfactants, can solve problems such as rosin resin acid molecules being easily oxidized
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
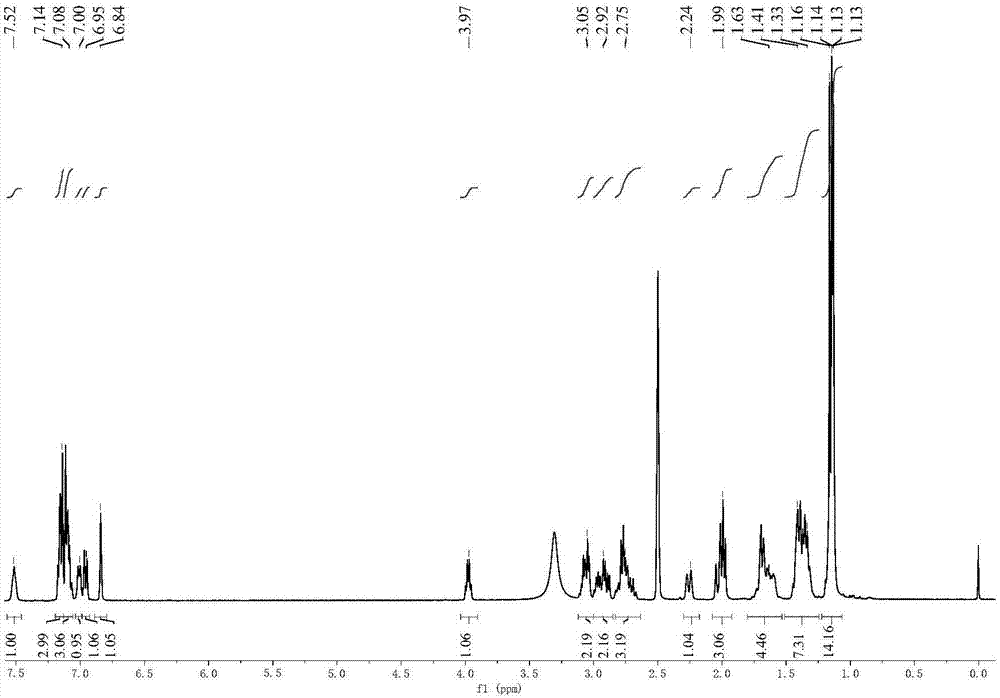
[0026] Example 1: Synthesis of Compound 1: Dissolve 6-aminocaproic acid methyl ester hydrochloride (32.7g, 0.18mol) in dichloromethane, add triethylamine (72.8g, 0.72mol), 4-di Methylaminopyridine (DMAP) (0.8g, 0.006mol) and dehydroabietic acid (48g, 0.16mol), then add 2-(7-benzotriazole oxide)-N,N,N',N'- Tetramethyluronium hexafluorophosphate (HATU) (76.0g, 0.20mol), the temperature was raised to 35°C, and reacted for 5h. After the reaction, extract with dichloromethane, wash the organic layer with deionized water for 5-6 times, and wash with anhydrous Na 2 SO 4 dry. After suction filtration, dichloromethane was removed by rotary evaporation. The product was purified by column chromatography (eluent: petroleum ether: ethyl acetate = 2:1) to obtain a colorless viscous liquid.
Embodiment 2
[0027] Example 2: Synthesis of R-6: Add NaOH (5.2g, 0.13mol) and 200mL of ethanol to a 500mL single-necked bottle, stir until NaOH is completely dissolved, then add compound 1 (47.0g, 0.11mol), and heat up to 70°C, react for 1.5h. After the reaction was completed, it was cooled to room temperature, a solid was precipitated, and R-6 was obtained by suction filtration.
Embodiment 3
[0028] Example 3: Synthesis of Compound 2: Dissolve R-6 (38.3g, 0.088mol) in water, add dilute HCl to make the solution acidic, extract with ethyl acetate, wash the organic layer with deionized water 5-6 times and use Anhydrous MgSO 4 After drying and suction filtration, ethyl acetate was removed by rotary evaporation to obtain compound 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com