Fluorescent probe for detecting activity of proline isomerase and preparation method and application thereof
A technology of fluorescent probes and isomerases, applied in peptide preparation methods, biochemical equipment and methods, luminescent materials, etc., can solve problems such as limited application range, inability to use living cells and in vivo detection, and achieve detection methods Simple, good probe stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1: the synthesis of probe molecule Ia
[0022]
[0023] "N-Fmoc-phenylalanyl-p-nitroaniline" was removed from the Fmoc protecting group and dissolved in dry dichloromethane methane solution, and an equivalent amount of "N,N-dimethylnaphthoic acid-labeled alanine -Phenylalanine-proline tripeptide chain, and equivalent EDC, HOBt condensation, the product is separated by preparative liquid phase to obtain the compound shown in formula Ia. MS: 798.3550.
Embodiment 2
[0024] Embodiment 2: the synthesis of probe molecule Ib
[0025]
[0026] "N-Fmoc-phenylalanyl-p-nitroaniline" was removed from the Fmoc protecting group and dissolved in dry dichloromethane methane solution, and an equivalent amount of "N,N-dimethylnaphthoic acid-labeled alanine - Glycine-proline tripeptide chain, and equivalent EDC, HOBt condensation, the product is separated by preparative liquid phase to obtain the compound shown in formula Ia. MS: 722.3333.
Embodiment 3
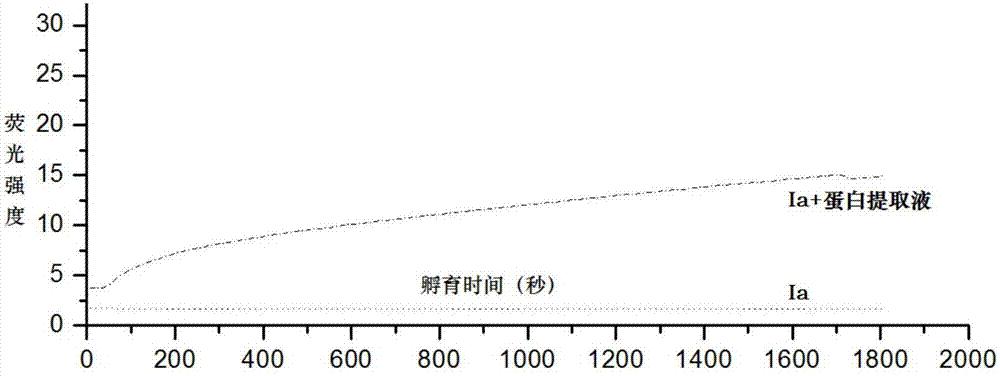
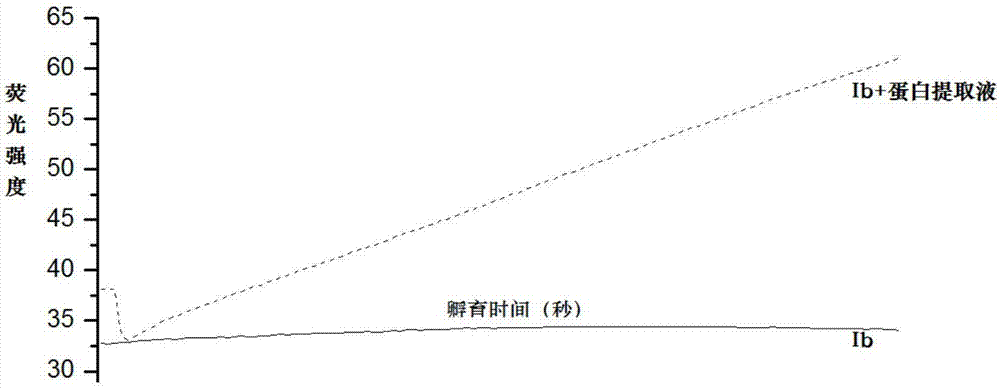
[0027] Embodiment 3: Probe molecule Ia detects the proline isomerase activity in the HBVP cell protein extract
[0028] Preparation of cell protein extract: Prepare cell lysate (10mM Tris, 150mM NaCl, 1mM EDTA, pH7.5), add 100μl lysate to each bottle of cells, and add corresponding protease inhibitor cocktail at the same time, lyse on ice for 30min, and centrifuge at 12000g After 10 minutes, pipette the supernatant into a new EP tube, use a kit (Bio-Rad, Cat. No. 5000111) for quantitative analysis of the protein extract, and finally supplement the lysate until the final concentration of the protein extract is 1 μg / μl.
[0029] Add Ia to the phosphate buffered saline solution to make the final concentration 10 micromoles, add 20 microliters of protein extract, and record the fluorescence emission intensity at 460nm with time under the excitation condition of 363nm with a fluorescence spectrophotometer. The experimental results show that the addition of protein extract can incre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com