Method for synthesizing polythioether
A synthesis method and technology of polythioether, applied in the field of polythioether polymers, can solve the problems of toxic polymer degradation reactions, etc., and achieve the effect of simple and easy preparation method and strong practicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Example 1, the stepwise addition reaction of 1,4-xylylene mercaptan and diethylene glycol divinyl ether catalyzed by p-methoxybenzaldehyde (wherein the molar ratio of the two monomers is 1:1)
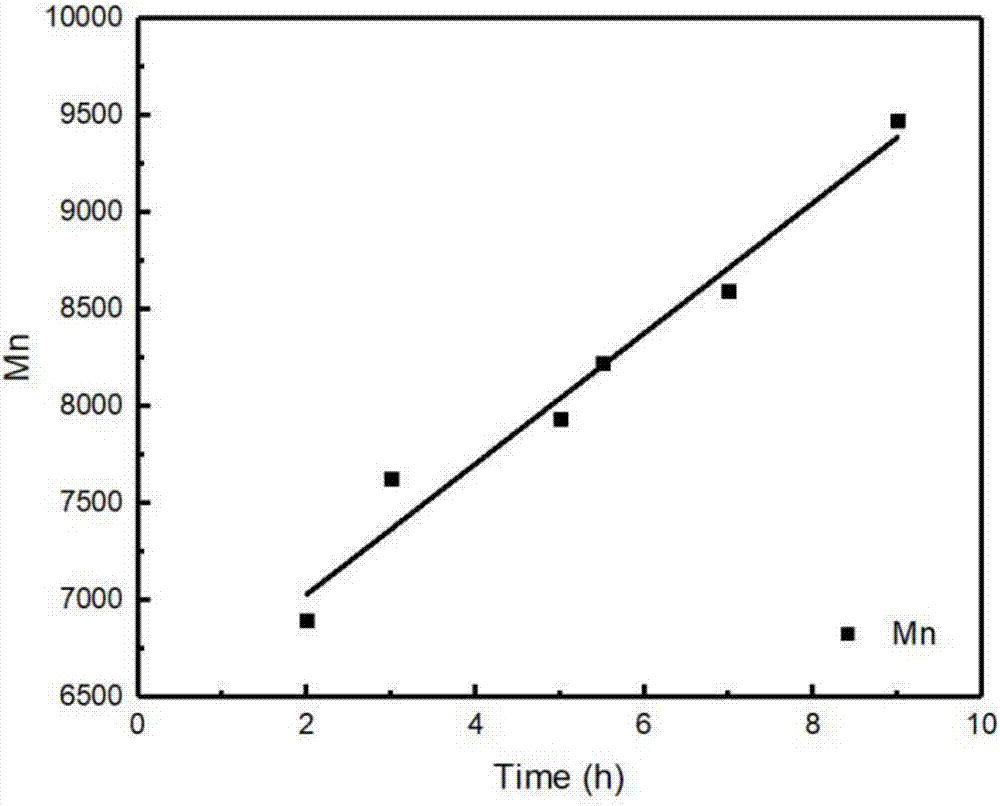
[0017] Add diethylene glycol divinyl ether (1.44g), 1,4-benzenedimethyl mercaptan (1.56g), anisole solution (3g), p-methoxybenzaldehyde ( 0.249g), filled with argon gas after refrigerated pumping to remove oxygen, and stirred at room temperature to make the reaction between two 23W compact energy-saving lamps (that is, the light source is in the wavelength range of 420nm, and the light intensity is 600~1000μWcm -2 ) under irradiation conditions. The polymer molecular weight increases linearly with time ( figure 1 ). After 4 hours of reaction, the monomer conversion rate reached 89%, and the polymer product M n =9600 g / mol, PDI = 1.78.
Embodiment 2
[0020] Embodiment two, the photopolymerization reaction of 1,4-xylylene mercaptan and diethylene glycol divinyl ether catalyzed by p-methoxybenzaldehyde (wherein 1,4-xylylene mercaptan is excessive)
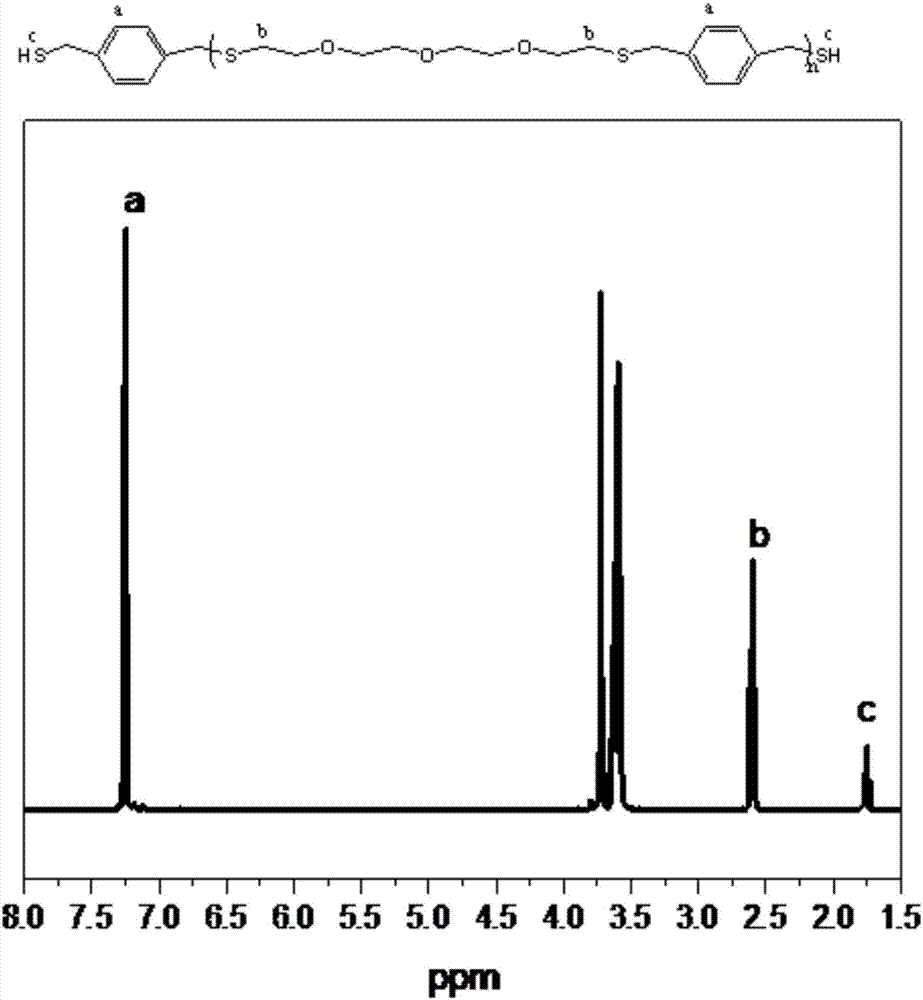
[0021] Add diethylene glycol divinyl ether (1.10g), 1,4-benzenedimethyl mercaptan (1.90g), anisole solvent (3g), p-methoxybenzaldehyde ( 0.124g). Refer to Example 1 for other operations (i.e., charge into argon gas after deoxygenation by freezing and pumping, stir at room temperature, and make the reaction in two 23W compact energy-saving lamps (light source 420nm wavelength range, light intensity is 600~1000μW cm -2 ) under the irradiation conditions, and the following examples omit the description and are all the same). After 7 hours of reaction, the monomer conversion rate reached 71%, and the polymer product M n = 4200 g / mol, PDI = 1.20. Proton NMR spectrum ( figure 2 ) showed that the reaction produced a narrow distribution of α, ω-dithiol-terminated telechelic polymers...
Embodiment 3
[0022] Embodiment three, the photopolymerization reaction of the mercapto-terminated telechelic polymer and diethylene glycol divinyl ether catalyzed by p-methoxybenzaldehyde
[0023] In a 25mL round-bottomed flask, add α,ω-dithiol-terminated oligomers (M n =4200g / mol, PDI=1.20), diethylene glycol divinyl ether, p-methoxybenzaldehyde (0.249g), anisole solvent (3g). The molar ratio of the telechelic polymer to the diethylene glycol divinyl ether monomer is 1:1, and the total concentration of the two monomers in the solvent is 50%. Refer to Embodiment 1 for other operations. After 12 hours of reaction, the monomer conversion rate reached 87%, and the polymer product M n = 16200 g / mol, PDI = 1.79. It is proved that the telechelic polymer is still active, and can continue to carry out addition reaction with divinyl monomer under the action of catalyst.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com