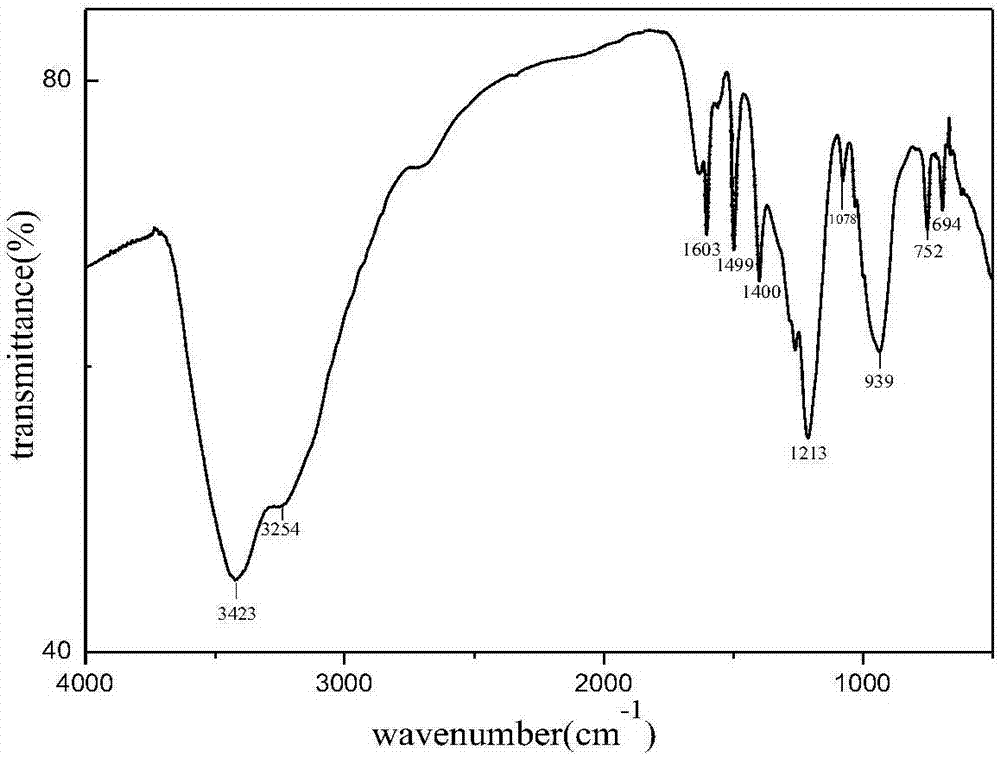
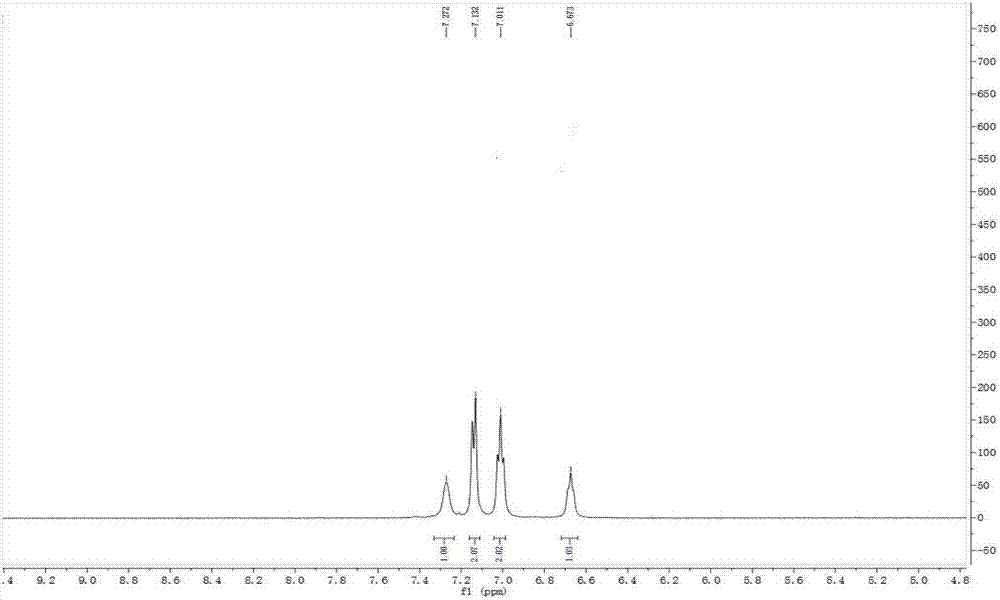
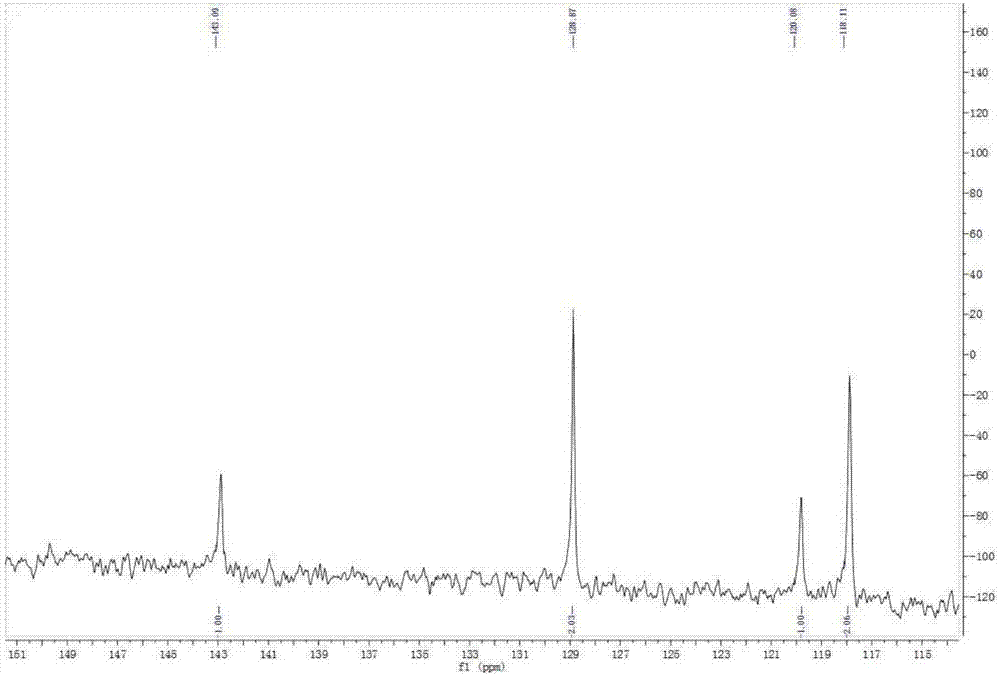
Phenyl-modified polyamino-cyclotriphosphazene and preparation method thereof
A technology of polyaminocyclotriphosphazene and aminocyclotriphosphazene, which is applied in the field of phenyl-modified polyaminocyclotriphosphazene and its preparation, can solve problems such as poor thermal stability and limitations, and achieve thermal stability improvement, Water-solubility and hygroscopicity decrease, phosphorus yield increase effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] The preparation technology of phenyl modified polyaminocyclotriphosphazene comprises the steps:
[0047] (1) Condensation: Add 17.4g of hexachlorocyclotriphosphazene, 6.1g of aniline, 6.6g of triethylamine, and 60g of chlorobenzene into a 250mL three-necked flask equipped with a thermometer, mechanical stirring, and reflux condenser, and pass the oil under stirring. The material was heated in a bath to 90°C and reacted at this temperature for 8h. Cool to room temperature, take a sample and analyze the free chlorine content, and calculate the degree of substitution of chlorine in the hexachlorocyclotriphosphazene (for the percentage of free chlorine mass and total chlorine mass in the sample) according to the free chlorine content. After analysis, the substitution degree of chlorine is 17.5%, which means that about 1.05 chlorine atoms in each phosphazene ring are replaced by anilino groups.
[0048] (2) Ammonification: The above materials are further cooled to about 0°C...
Embodiment 2
[0056] The preparation technology of phenyl modified polyaminocyclotriphosphazene comprises the steps:
[0057] (1) Condensation: Add 17.4g of hexachlorocyclotriphosphazene, 6.1g of aniline, 6.6g of triethylamine, and 60g of chlorobenzene into a 250mL three-necked flask equipped with a thermometer, mechanical stirring, and reflux condenser, and pass the oil under stirring. The material was heated in a bath to 90 °C and reacted at this temperature for 6 h. Cool to room temperature, take a sample to analyze the free chlorine content, and calculate the substitution degree of chlorine in hexachlorocyclotriphosphazene according to the free chlorine content. After analysis, the substitution degree of chlorine is 13.6%, which means that about 0.82 chlorine atoms in each phosphazene ring are substituted by anilino groups.
[0058] (2) Ammonification: The above materials are further cooled to about 0°C with an ice-salt bath, and ammonia gas is introduced at a uniform speed for 18 hour...
Embodiment 3
[0063] The preparation technology of phenyl modified polyaminocyclotriphosphazene comprises the steps:
[0064] (1) Condensation: Add 17.4g of hexachlorocyclotriphosphazene, 6.1g of aniline, 6.6g of triethylamine, and 60g of chlorobenzene into a 250mL three-necked flask equipped with a thermometer, mechanical stirring, and reflux condenser, and pass the oil under stirring. The material was heated in a bath to 90° C. and reacted at this temperature for 10 h. Cool to room temperature, take a sample to analyze the free chlorine content, and calculate the substitution degree of chlorine in hexachlorocyclotriphosphazene according to the free chlorine content. After analysis, the substitution degree of chlorine is 17.6%, which means that about 1.05 chlorine atoms in each phosphazene ring are replaced by anilino groups.
[0065] (2) Ammonification: The above materials are further cooled to about 0°C with an ice-salt bath, and ammonia gas is introduced at a uniform speed for 18 hours...
PUM
| Property | Measurement | Unit |
|---|---|---|
| water absorption | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com