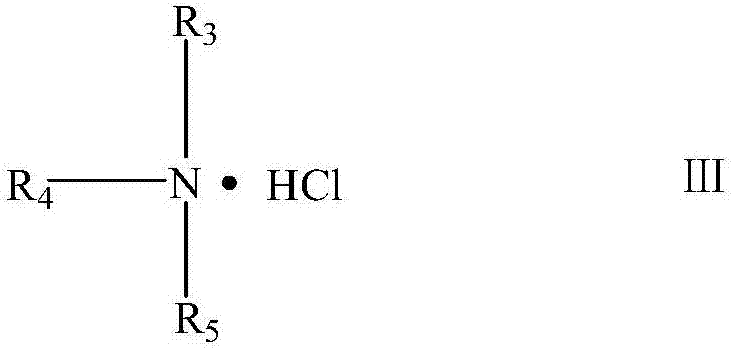Asymmetric gemini organosilicon quaternary ammonium salt antibacterial agent and preparation method thereof
An organosilicon quaternary ammonium salt, asymmetric technology, applied in botany equipment and methods, fungicides, organic chemistry, etc., can solve problems such as structural asymmetry, achieve long-lasting antibacterial performance, wide antibacterial spectrum, and wide application range Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
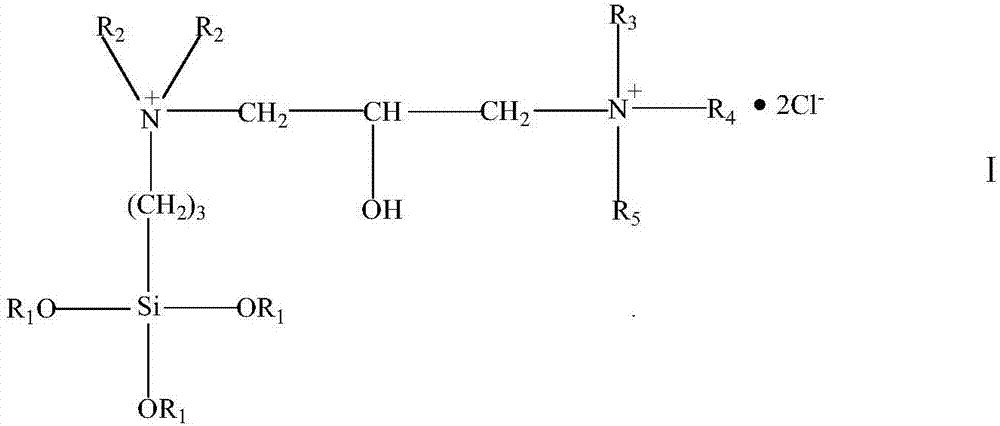
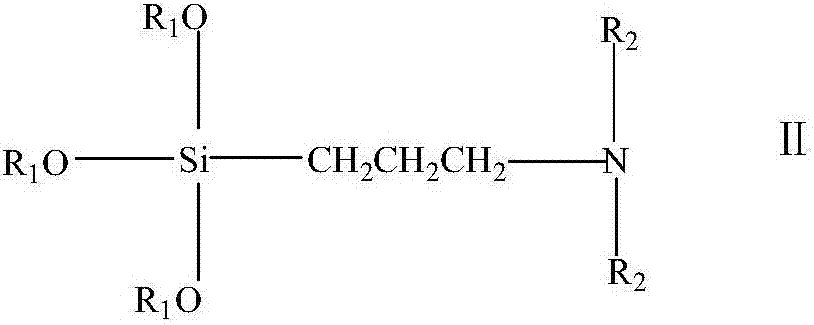
[0020] Dissolve 0.1mol of N,N-dimethylaminopropyltrimethoxysilane in 30g of absolute ethanol, stir and raise the temperature to 60°C, add 0.15mol of epichlorohydrin dropwise, finish adding dropwise in 30min, and continue the reaction for 5h After the reaction, the solvent and epichlorohydrin are recovered under reduced pressure to obtain a light yellow transparent paste, which is the epoxy-based organosilicon quaternary ammonium salt intermediate. Then, 0.1 mol trimethylamine hydrochloride and 0.1 mol epoxy organosilicon quaternary ammonium salt intermediate were dissolved in anhydrous tetrahydrofuran, reacted at 70°C for 6 hours, depressurized distillation, and vacuum dried to obtain light yellow Asymmetric gemini silicone quaternary ammonium antibacterial agent.
Embodiment 2
[0022] Dissolve 0.05 mol of N,N-dimethylaminopropyltrimethoxysilane in 20 grams of anhydrous isopropanol and stir to raise the temperature to 60°C, add 0.65 mol of epichlorohydrin dropwise, finish adding dropwise in 30 minutes, continue After the reaction for 4 hours, the solvent and epichlorohydrin were recovered under reduced pressure after the reaction, and a light yellow transparent paste was obtained, which was the intermediate of epoxy organosilicon quaternary ammonium salt. Then, dissolve 0.05mol of dodecyl dimethyl hydrochloride and 0.05mol of epoxy organosilicon quaternary ammonium salt intermediate in anhydrous tetrahydrofuran, react at 70°C for 6h, distill under reduced pressure, and dry in vacuo , to obtain a light yellow asymmetric gemini organosilicon quaternary ammonium antibacterial agent.
Embodiment 3
[0024] Dissolve 0.05 mol of N,N-dimethylaminopropyltrimethoxysilane in 20 grams of anhydrous methanol and stir to raise the temperature to 60°C, add 0.6 mol of epichlorohydrin dropwise, finish adding dropwise in 30 minutes, and continue the reaction for 6 hours After the reaction, the solvent and epichlorohydrin are recovered under reduced pressure to obtain a light yellow transparent paste, which is the epoxy-based organosilicon quaternary ammonium salt intermediate. Then dissolve 0.05mol hexadecyl dimethyl hydrochloride and 0.05mol epoxy group organosilicon quaternary ammonium salt intermediate in anhydrous acetone, react at 60°C for 10h, distill under reduced pressure, and dry in vacuo , to obtain a light yellow asymmetric gemini organosilicon quaternary ammonium antibacterial agent.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com