Preparation technology for 1-(3-amino-4-pyridyl) aceton
A preparation process and aminopyridine technology are applied in the field of preparation technology of 1-ethyl ketone, which can solve the problems of high price and high production cost, and achieve the effects of cheap raw materials, mild and easy-to-control operation conditions, and favorable industrial application.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
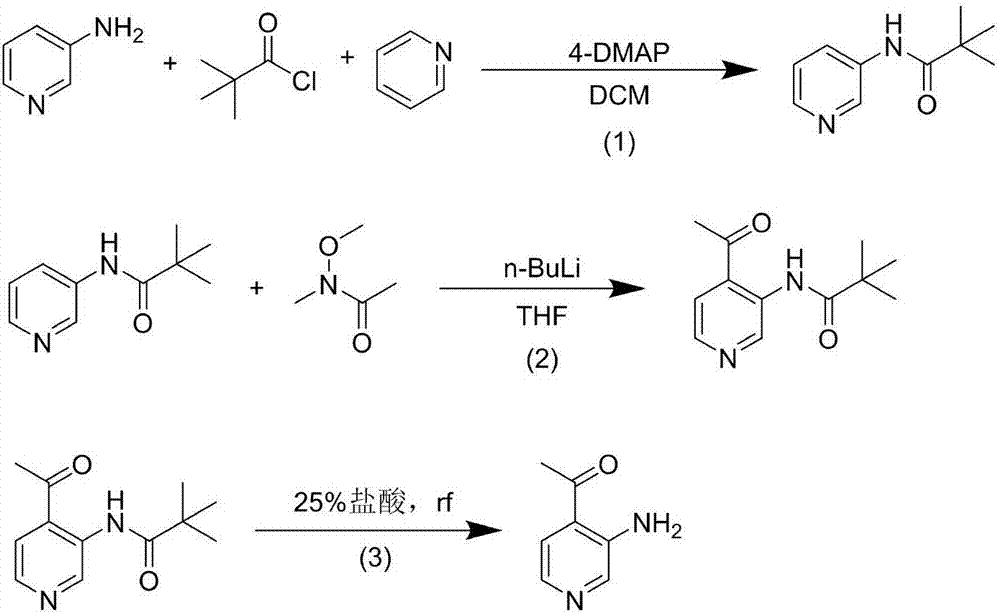
[0012] A. Preparation of 3-pivalamidopyridine
[0013] In a three-necked flask, 3-aminopyridine (20.0g, 212.5mmol), 4-dimethylaminopyridine (4-DMAP) (0.3g, 2.1mmol) and pyridine (33.6g, 425.0mmol) were dissolved in anhydrous di In methyl chloride (200mL), pivaloyl chloride (28.2g, 233.7mmol) was slowly added dropwise under ice-cooling, and reacted at 0°C for 2h. After the reaction was completed, the reaction solution was poured into ice water, quenched with 1N hydrochloric acid, extracted with dichloromethane (250mL×3), the organic phase was collected, washed with saturated brine (200mL×1), and the solvent was evaporated under reduced pressure to obtain 3- 36.2 g of pivalamidopyridine, yield 95.5%.
[0014] B. Preparation of N-(4-ethylpyridin-3-yl)trimethylacetamide
[0015] In a three-necked flask, 3-pivalamidopyridine (20.0 g, 112.2 mmol) was dissolved in anhydrous tetrahydrofuran (200 mL), cooled to -78°C under nitrogen protection, and n-butyllithium (17.3 g, 269.3mmol),...
Embodiment 2
[0020] A. Preparation of 3-pivalamidopyridine
[0021] In a three-necked flask, 3-aminopyridine (20.0g, 212.5mmol), 4-dimethylaminopyridine (4-DMAP) (0.3g, 2.1mmol) and pyridine (33.6g, 425.0mmol) were dissolved in anhydrous di To methyl chloride (200 mL), slowly add pivaloyl chloride (25.6 g, 212.5 mmol) dropwise under ice-cooling, and react at 30°C for 1 h after the drop is complete. After the reaction was completed, the reaction solution was poured into ice water, quenched with 1N hydrochloric acid, extracted with dichloromethane (250mL×3), the organic phase was collected, washed with saturated brine (200mL×1), and the solvent was evaporated under reduced pressure to obtain 3- 33.8 g of pivalamidopyridine, yield 89.2%.
[0022] B. Preparation of N-(4-ethylpyridin-3-yl)trimethylacetamide
[0023] In a three-necked flask, 3-pivalamidopyridine (20.0 g, 112.2 mmol) was dissolved in anhydrous tetrahydrofuran (200 mL), cooled to -78°C under nitrogen protection, and n-butyllithi...
Embodiment 3
[0028] A. Preparation of 3-pivalamidopyridine
[0029] In a three-necked flask, 3-aminopyridine (20.0g, 212.5mmol), 4-dimethylaminopyridine (4-DMAP) (2.6g, 21.2mmol) and pyridine (168.1g, 2124.9mmol) were dissolved in anhydrous di Pivaloyl chloride (51.3 g, 425.0 mmol) was slowly added dropwise to methyl chloride (200 mL) in an ice bath, and reacted at -10°C for 2 h. After the reaction was completed, the reaction solution was poured into ice water, quenched with 1N hydrochloric acid, extracted with dichloromethane (250mL×3), the organic phase was collected, washed with saturated brine (200mL×1), and the solvent was evaporated under reduced pressure to obtain 3- 35.1 g of pivalamidopyridine, yield 92.5%.
[0030] B. Preparation of N-(4-ethylpyridin-3-yl)trimethylacetamide
[0031] In a three-necked flask, 3-pivalamidopyridine (20.0 g, 112.2 mmol) was dissolved in anhydrous tetrahydrofuran (200 mL), cooled to -78°C under nitrogen protection, and n-butyllithium (21.6 g, 336.6m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com