Budesonide nanocrystal, preparation method and application in atomization inhalation administration
A nanocrystal, atomization inhalation technology, applied in nanotechnology, nanotechnology, nano-optics and other directions, can solve the problems of clogging the vibrating mesh, not recommended, etc., to improve compliance, relieve asthma symptoms, and achieve the effect of small medication volume
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
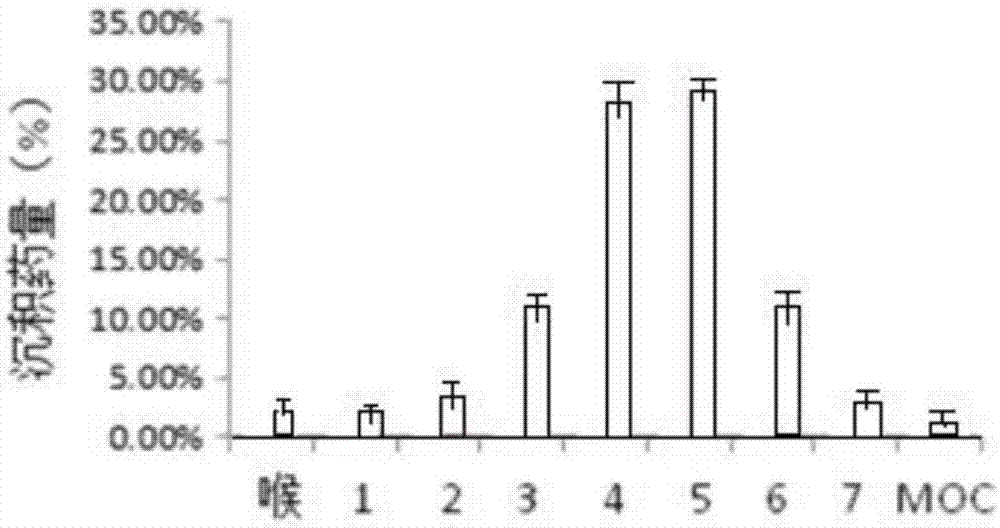
Image
Examples
Embodiment 1
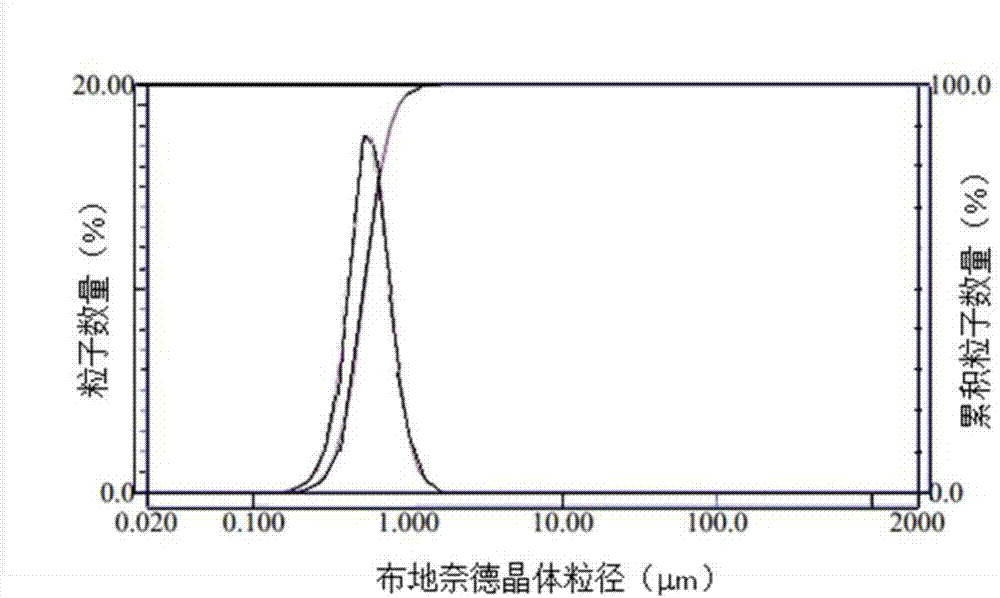
[0033] The preparation of embodiment 1 budesonide nanocrystal
[0034] Dissolve 0.5 g of budesonide in 20 ml of ethanol, filter through a 0.22 μm microporous membrane to obtain sterile solution 1, and pre-cool to 0°C for later use;
[0035] Dissolve 0.2g of Tween 80 in water for injection, filter through a 0.22μm microporous membrane to obtain sterile solution 2, and pre-cool to 0°C for later use;
[0036] Add the pre-cooled solution 1 to the pre-cooled solution 2 at -15°C under stirring, continue to stir for 4 minutes, collect budesonide nanocrystals after centrifugation, and wash with a small amount of water for injection to obtain the budesonide Ned nanocrystals.
Embodiment 2
[0037] The preparation of embodiment 2 budesonide nanocrystals
[0038] Dissolve 5 g of budesonide in 50 ml of ethanol, filter through a 0.22 μm microporous membrane to obtain sterile solution 1, and pre-cool to 3°C for use;
[0039] Dissolve 2 g of Poloxamer 188 in water for injection, filter through a 0.22 μm microporous membrane to obtain sterile solution 2, and pre-cool to 3°C for use;
[0040] Add the pre-cooled solution 1 to the pre-cooled solution 2 at -20°C under stirring, continue stirring for 4 minutes, collect budesonide nanocrystals after centrifugation, and wash with a small amount of water for injection to obtain the budesonide Ned nanocrystals.
Embodiment 3
[0041] Example 3 Budesonide atomized inhalation nanosuspension
[0042] The prescription of budesonide nebulized inhalation nanosuspension is as follows:
[0043]
[0044] Preparation method:
[0045] Dissolve the prescribed amount of Tween 80, sodium chloride, and EDTA-2Na in water for injection, adjust the pH to 4.0 with dilute hydrochloric acid, and filter through a 0.22 μm microporous membrane to obtain a sterile solution;
[0046] Add budesonide nanocrystals to the above sterile solution, and add water for injection to a prescribed volume of 1000ml;
[0047] The above-mentioned nanocrystal suspension is divided into low-density polyethylene small tubes, sealed, and 0.2ml per tube is obtained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com