Pathogenic gene mutation of hypothyroidism and diagnostic reagent based on same
A technology of thyroid function, diagnostic kit, applied in the field of medical diagnosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1 sample acquisition
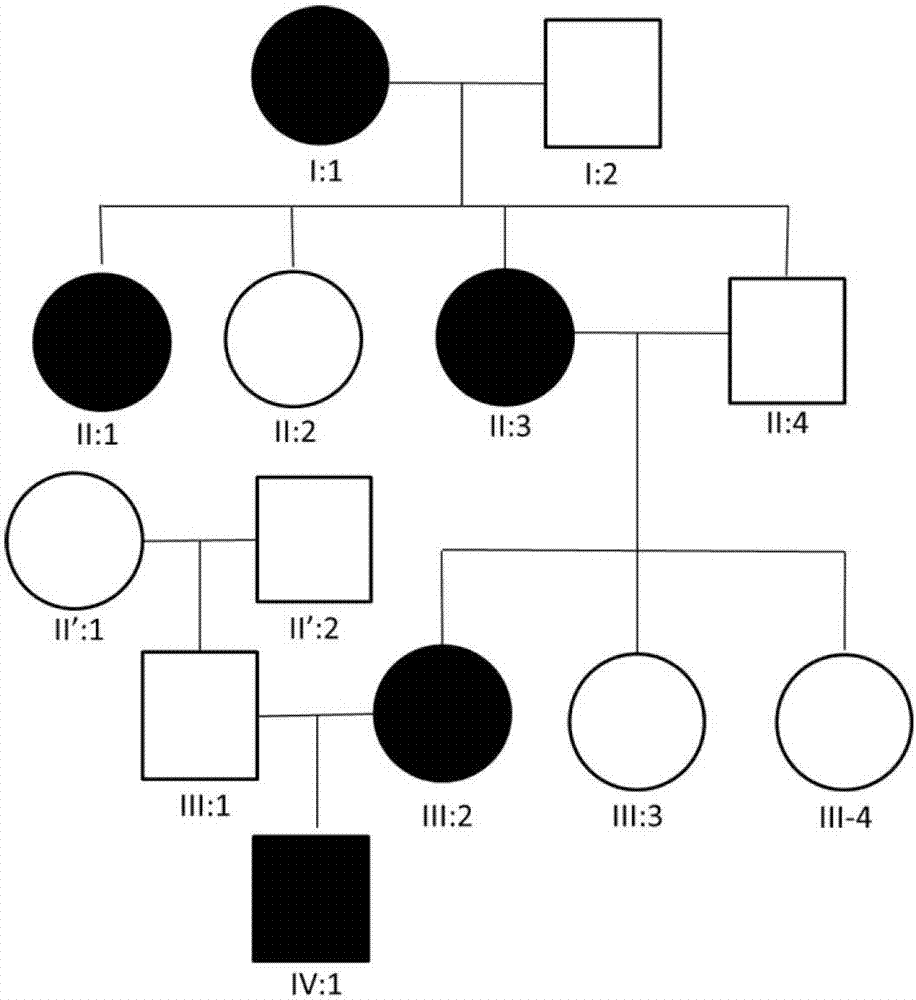
[0042] The inventor discovered a family with four generations of hypothyroidism (referred to as hypothyroidism), and the clinical information of some members of the family with overt hypothyroidism is shown in Table 1. There are patients with hypothyroidism in the four generations of the family, and it is speculated that the disease is a dominant inheritance; in addition, there are females and males in the patients, so it is speculated that the disease is autosomal inheritance, which has nothing to do with gender. figure 1 Pedigrees for overt hypothyroidism are shown, where ● or ■ indicate affected individuals.
[0043] Diagnostic criteria:
[0044] You can refer to the 2007 edition of "Guidelines for Diagnosis and Treatment of Thyroid Diseases in China":
[0045] (1) Clinical manifestations: early stage patients with mild disease may have no specific symptoms. Typical patients experience chills, fatigue, swelling in hands and feet, ...
Embodiment 2
[0053] Example 2 Exon sequencing
[0054] 1. Instruments and equipment are shown in Table 2.
[0055] Table 2 Instruments and equipment
[0056]
[0057]
[0058] 2. Reagent consumables
[0059] Agilent SureSelect Human All Exon Kit (Agilent), Agilent DNA 1000Kit (Agilent), 1.5ml centrifuge tube (Eppendorf), 0.2ml centrifuge tube (Eppendorf), tip (Axygen), 96-well plate (Axygen), BigDye v3.1 (Thermo), absolute ethanol, POP7 gel (Thermo), capillary electrophoresis Buffer (Thermo), Sequence Standard (3500) (Thermo), blood genomic DNA extraction kit (TIANGEN), D2000 DNA Marker (TIANGEN), agarose (SunShineBio ), EB (Amresco).
[0060] 3. Reagent formula
[0061] 3.1 TBE electrophoresis solution
[0062] 5×TBE electrophoresis solution was prepared according to Table 3.
[0063] Table 3 Formula of 5×TBE electrophoresis solution
[0064] Reagent
volume
54g
7.5g
EDTA (pH 8.0, 0.5mol / L)
20mL
wxya 2 o
...
Embodiment 3
[0106] Example 3 Sanger sequencing verification
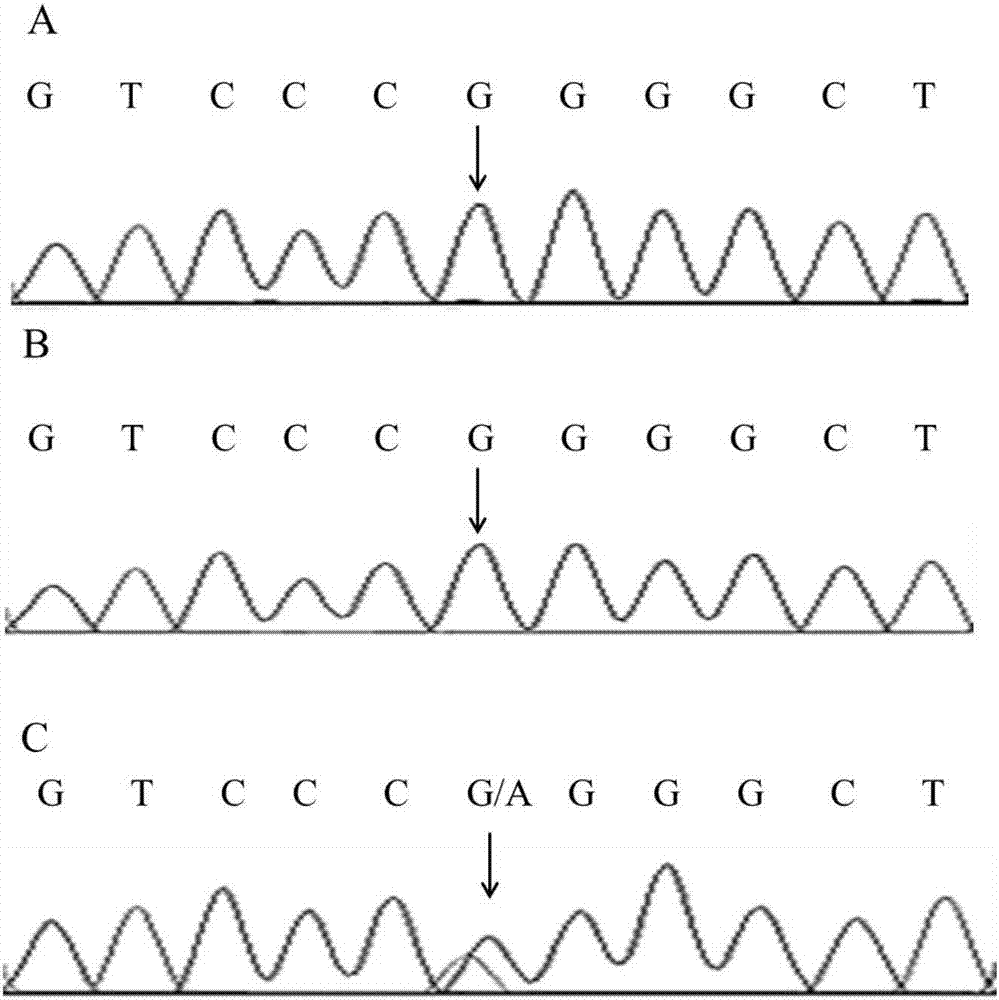
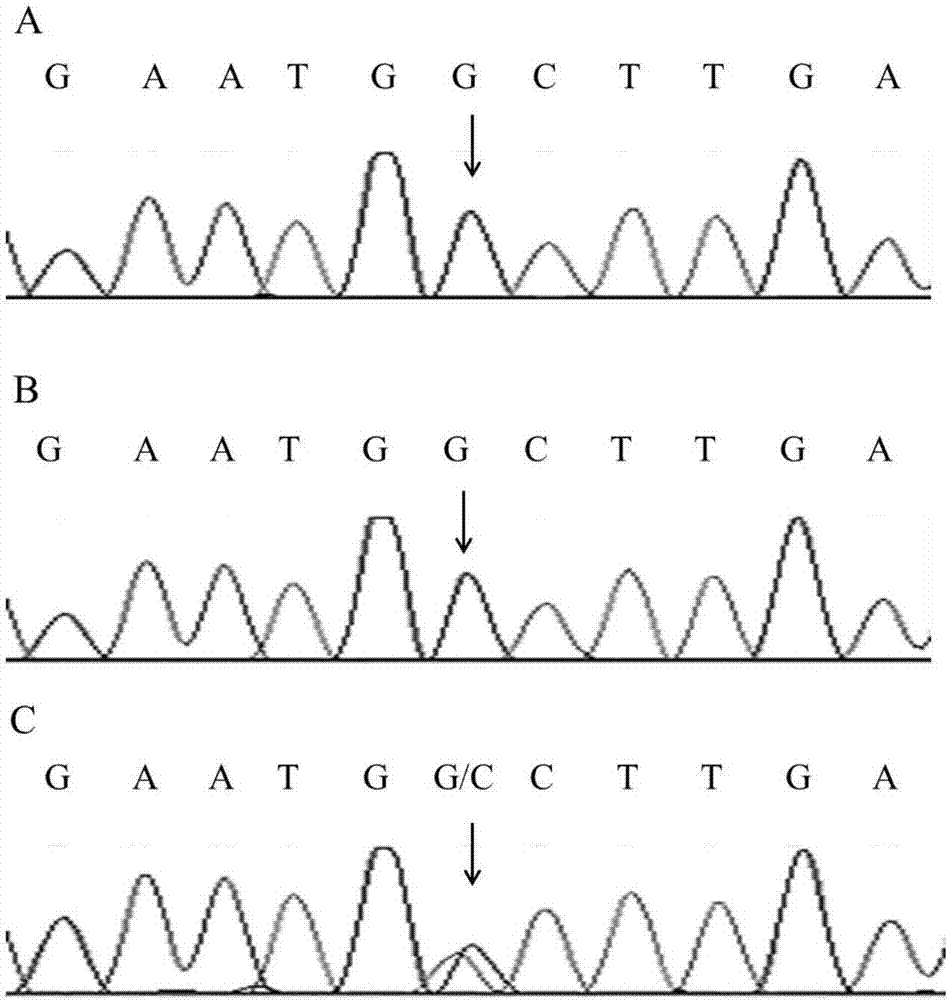
[0107] Because exome sequencing has a certain degree of false positives, we further used Sanger sequencing to detect CHRNA1:NM_000079:exon6:c.G605A:p.R202Q and TRPM8:NM_024080:exon12:c.G1442C:p. The G481A site was verified. The CHRNA1:NM_000079:exon6:c.G605A:p.R202Q site and TRPM8:NM_024080:exon12:c.G1442C were performed on the 5 patients, 8 normal persons in the family line and 100 normal persons outside the family line in Example 1 respectively :p.G481A site genotype detection.
[0108] The specific method steps are as follows:
[0109] (1) DNA extraction
[0110] Genomic DNA in peripheral blood leukocytes was extracted according to the method in Example 1.
[0111] (2) Primer design
[0112] Primers were designed with reference to the human genome sequence database hg19 / build36.3.
[0113] The primer sequences for the CHRNA1:NM_000079:exon6:c.G605A:p.R202Q site are as follows:
[0114] 5'-AAACCTCACTTCCTTTTCTCAGGA-3' (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com