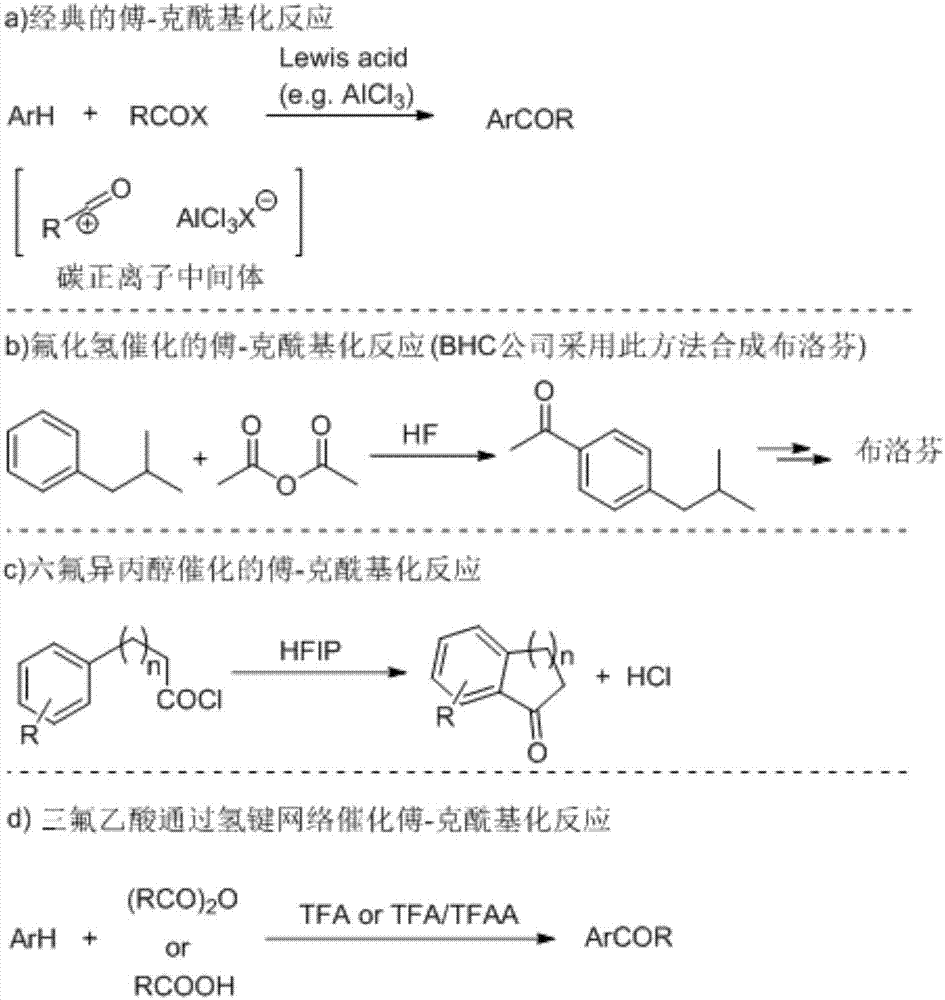
Friedel-Crafts acylation reaction catalyzed trifluoroacetic acid
A technology of trifluoroacetic acid and trifluoroacetic anhydride, which is applied in the field of Friedel-Crafts acylation reaction, can solve the problems that catalysts cannot be recycled well, environmental pollution, a large amount of aluminum salt waste liquid, etc., and achieves good application prospects and economy. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] By screening the reaction conditions:
[0019] entry
equiv of 2a
Yield b / %
1
2
TFA (1mL)
100
2
2
50:50 TFA / DCM
69
3
2
80:20 TFA / DCM
95
4
2
50:50 TFA / HFIP
14
5
2
80:20 TFA / HFIP
44
6
2
TFA (0.5mL)
95
7
2
TFA (0.8mL)
100(95 c )
8
1.2
TFA (0.8mL)
84
9
1.5
TFA (0.8mL)
96
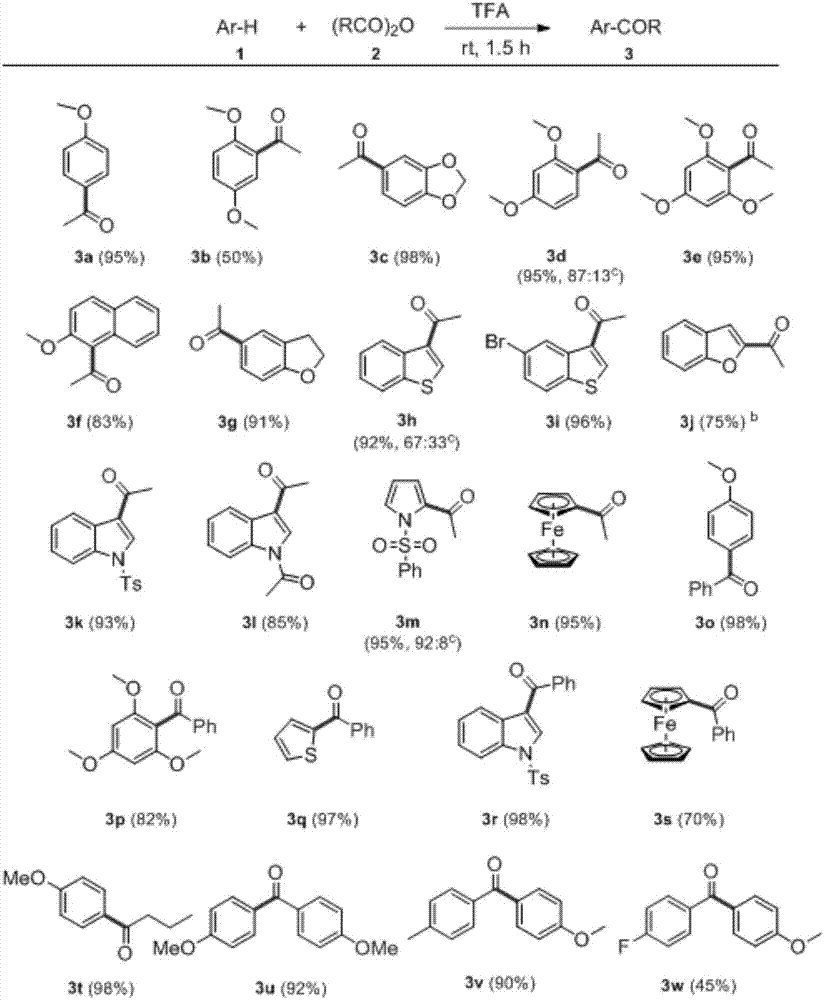
[0020] The best reaction conditions were obtained, that is, at room temperature, anisole (0.75mmol) and acetic anhydride (1.5mmol) were reacted in trifluoroacetic acid (0.8mL) for 1.5h to obtain the target product with a conversion rate of 100%. The applicable range and conversion rate of other substrates are as follows: figure 2 shown.
[0021]
Embodiment 2
[0023] By screening the reaction conditions:
[0024] entry
equiv of 2a
Yield b / %
1
1
TFA (1mL)
53
2
1.5
TFA (1mL)
62
3
2
TFA (1mL)
50
4
3
TFA (1mL)
62
5
1.5
TFA (2mL)
78(65 c )
6
1.5
TFA (3mL)
71
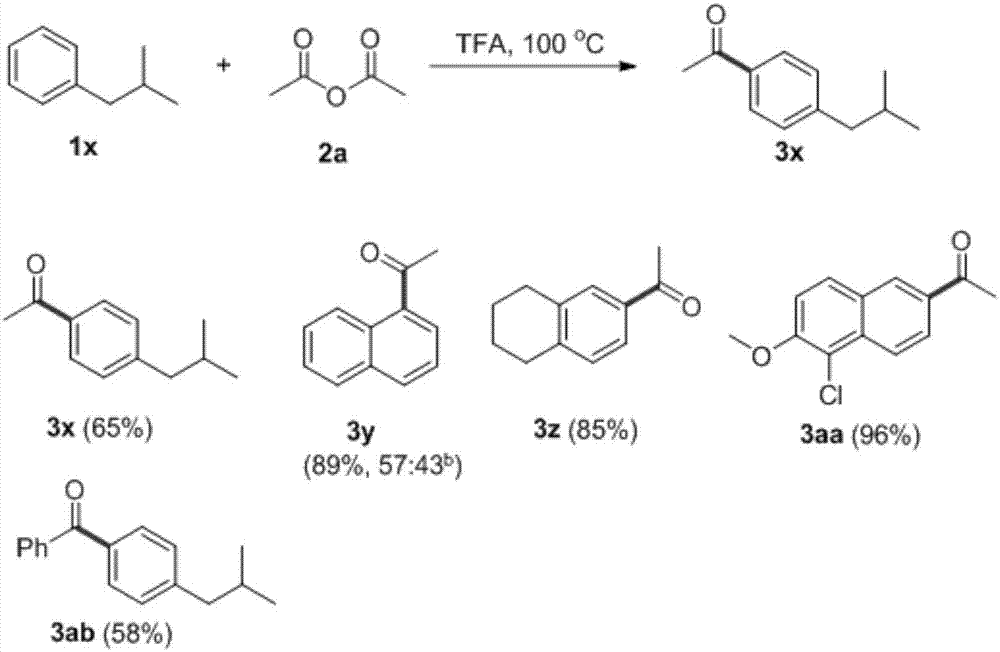
[0025] The optimal reaction conditions were obtained, that is, at 100°C, isobutylbenzene (0.75mmol) and acetic anhydride (1.125mmol) were reacted in trifluoroacetic acid (2mL) for 56h to obtain the target product with a conversion rate of 78%. The applicable range and conversion rate of other substrates are as follows: image 3 shown.
[0026]
Embodiment 3
[0028] By screening the reaction conditions:
[0029] entry
equiv of 1a
Yield b / %
1
1
Trifluoroacetate
48
2
1.2
Trifluoroacetate
95
3
1.5
Trifluoroacetate
98
4
2
Trifluoroacetate
98
[0030] The best reaction conditions were obtained, that is, at room temperature, anisole (1.125mmol), benzoic acid (0.75mmol), and trifluoroacetic anhydride (1.5mmol) were reacted in trifluoroacetic acid (0.8mL) for 12h to obtain the target Product, 98% conversion. The applicable range and conversion rate of other substrates are as follows: Figure 4 shown.
[0031]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com