Ionic type iridium complex with double phosphorescence emission properties as well as preparation method and application of ionic type iridium complex
A technology of phosphorescent emission and iridium complexes, which is applied in the direction of indium organic compounds, platinum group organic compounds, and compounds containing elements of Group 8/9/10/18 of the periodic table, etc., which can solve the problem of no concentration and difficulty in controlling the concentration of complexes , the concentration of the complex affects the detection results of the analyte, etc., to achieve the effects of reduced interference, accurate quantification, and reliable detection results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
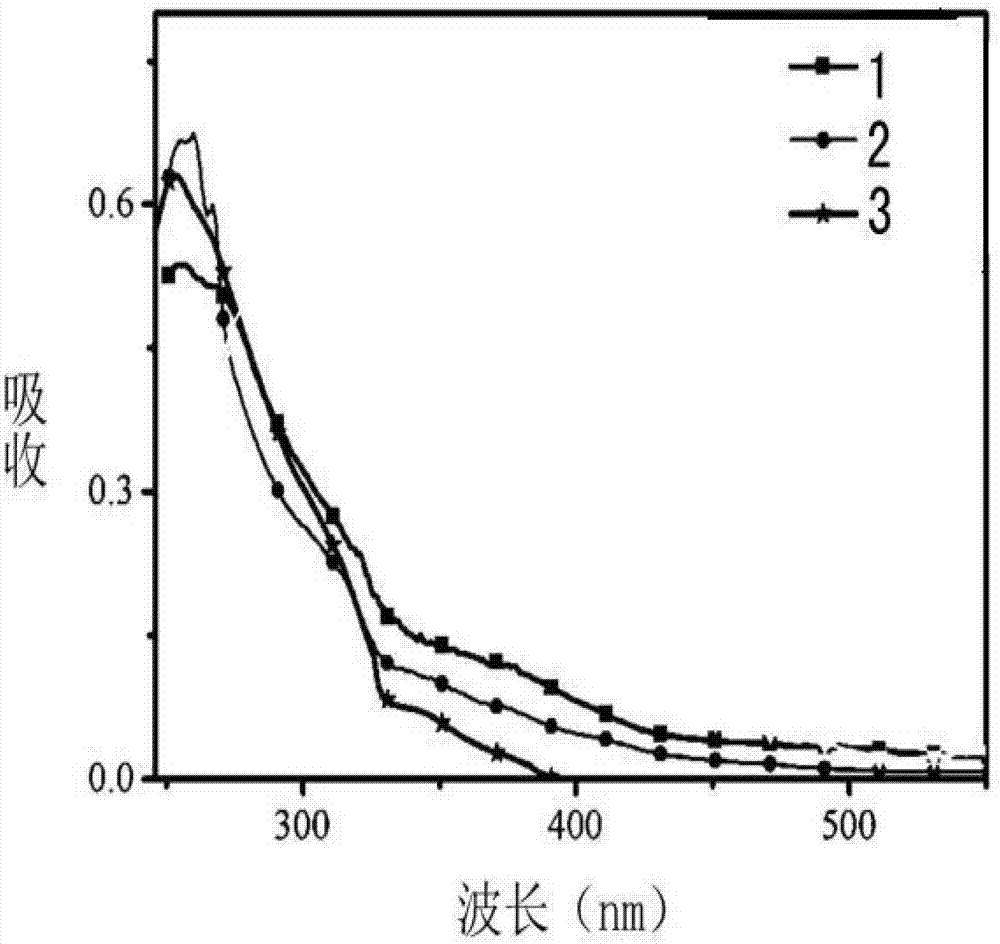
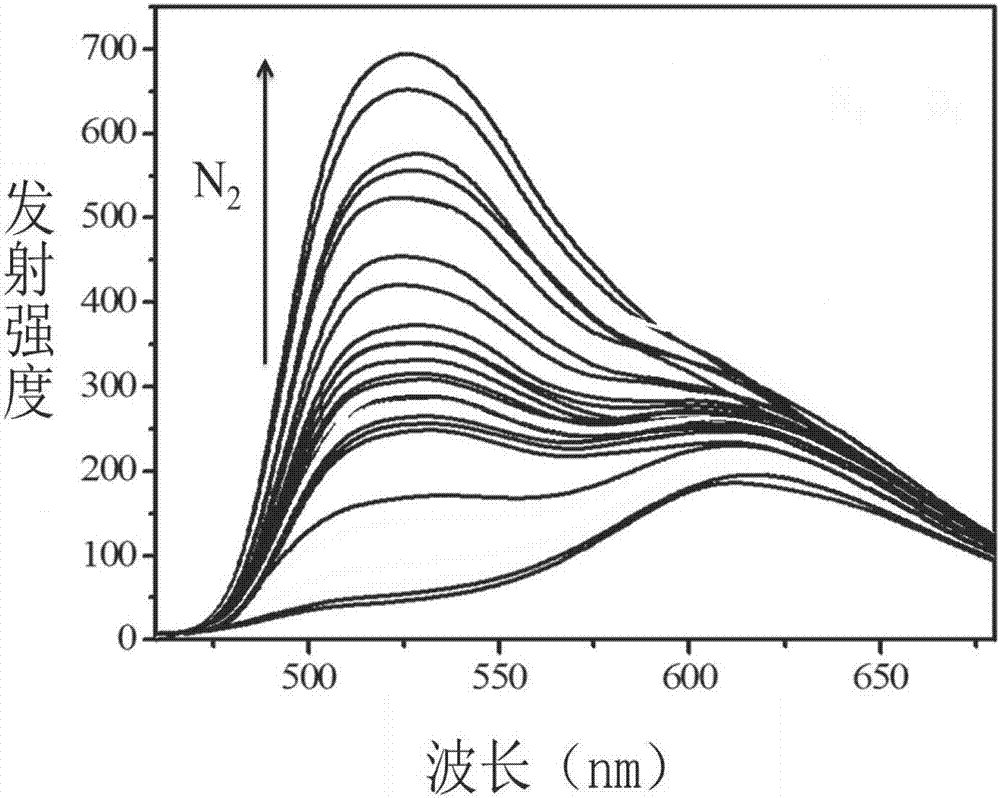
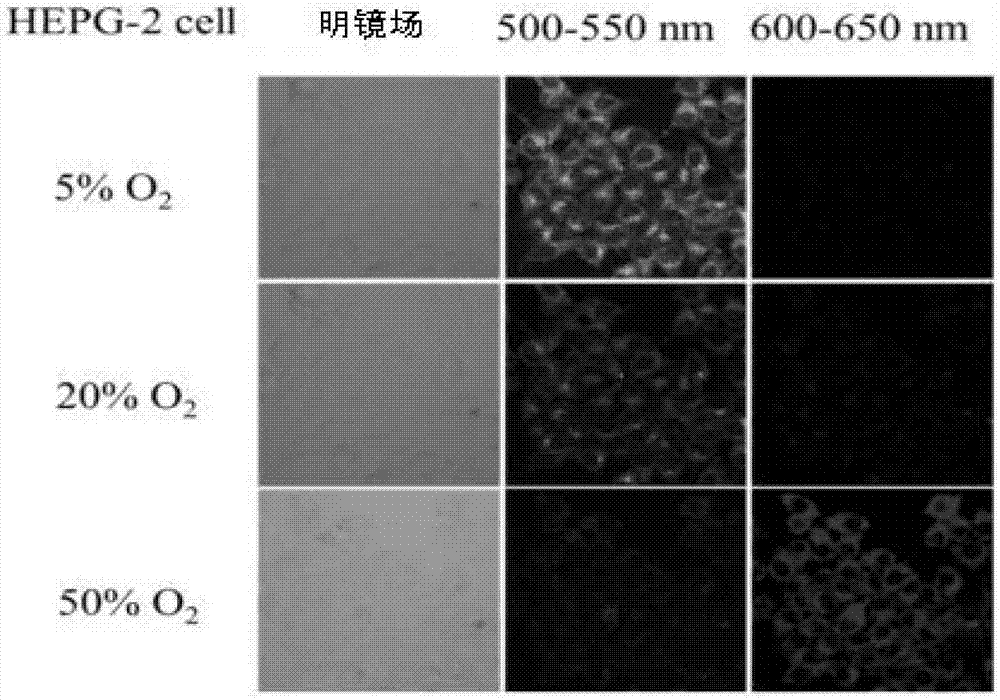
Image
Examples
Embodiment 1
[0037] (1) Synthesis of 4-methyl-4'-acetamide-2,2'-bipyridine
[0038]
[0039] Synthesis of 4-methyl-4'-formyl-2,2'-bipyridine: Weigh 5.27g (28mmol) of 4,4'-dimethyl-2,2'-bipyridine, SeO 2 3.84g (34.6mmol), add 260mL of 1,4-dioxane, vacuumize, react under nitrogen protection at 102°C for 24h, then weigh and filter hot; spin the filtrate to dryness, and dissolve it in 500mL of ethyl acetate, then Filtration again to remove insoluble solids gave a yellow solution, which was treated with 1.0 M Na 2 CO 3 (2×100mL) was extracted twice, and the organic phase was taken; then 0.3M (3×100mL) Na 2 S 2 o 5 Take the inorganic phase, use Na 2 CO 3 Adjust the pH value of the inorganic phase to 10, then use CH 2 Cl 2 Extraction (4×100 mL) and spin-drying afforded 2.5 g of white solid, yield 45%. 1 H NMR (400MHz, CDCl3, δ): 10.1(1H,s), 8.80(1H,d), 8.73(1H,s), 8.49(1H,d), 8.18(1H,s), 7.63(1H,d ), 7.11(1H,d), 2.37(3H,s).
[0040]Synthesis of 4-methyl-4'-carboxy-2,2'-bipyridine: W...
Embodiment 2
[0052] (1) Synthesis of 4,4'-dibutyramide-2,2'-bipyridine:
[0053]
[0054] 1) Synthesis of 4,4'-dicarboxy-2,2'-bipyridine: Weigh 1.0g (5.4mmol) of 4,4-dimethyl-2,2,-bipyridine into the reaction flask, drop slowly Add 18 mL of concentrated sulfuric acid and stir. During the process, a large amount of heat is released. After the solution is cooled to room temperature, 3.3 g (11.3 mmol) of potassium dichromate is added. At this time, the solution turns dark green, and then reacted at 65 ° C for 6 h. After the reaction is completed, cool to room temperature, add the reactant to 1000mL of water, at this time a large amount of white flocculent precipitates are produced, filter with suction, wash the white precipitate with water and methanol in sequence until the filtrate is clear, and then dry it in a vacuum oven at 60°C for 18 hours. Yield 98%.
[0055] Synthesis of 4,4'-dicarboxylate-2,2'-bipyridine: Weigh 500mg (2.05mmol) of 4,4'-dicarboxy-2,2'-bipyridine into the reaction ...
Embodiment 3
[0067] (1) Synthesis of 4,4'-dibutyramide-2,2'-bipyridine:
[0068]
[0069] 1) Synthesis of 4,4'-dicarboxy-2,2'-bipyridine: Weigh 1.0g (5.4mmol) of 4,4-dimethyl-2,2,-bipyridine into the reaction flask, drop slowly Add 18 mL of concentrated sulfuric acid and stir. During the process, a large amount of heat is released. After the solution is cooled to room temperature, 3.3 g (11.3 mmol) of potassium dichromate is added. At this time, the solution turns dark green, and then reacted at 65 ° C for 6 h. After the reaction is completed, cool to room temperature, add the reactant to 1000mL of water, at this time a large amount of white flocculent precipitates are produced, filter with suction, wash the white precipitate with water and methanol in sequence until the filtrate is clear, and then dry it in a vacuum oven at 60°C for 18 hours. Yield 98%.
[0070] Synthesis of 4,4'-dicarboxylate-2,2'-bipyridine: Weigh 500mg (2.05mmol) of 4,4'-dicarboxy-2,2'-bipyridine into the reaction ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com