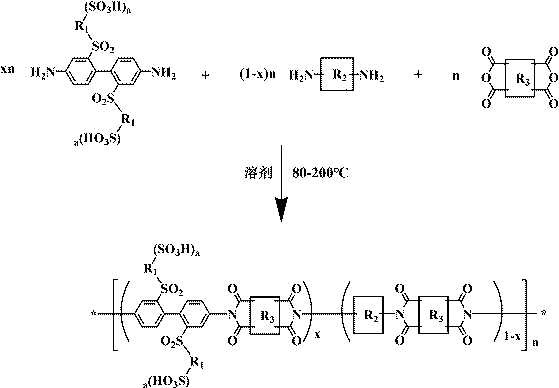
Sulfuryl bridge connection side chain sulfonated polyimide, preparation method thereof and application
A technology of sulfonated polyimide and sulfonated polyimide copolymer, which is applied in electrochemical generators, final product manufacturing, sustainable manufacturing/processing, etc., can solve the problem of reducing the acidity and difficulty of sulfonic acid groups Phase separation structure, affecting performance and other issues, to achieve the effect of high molecular weight, simple preparation process, and easy control of reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1: Synthesis of a sulfone-bridged side-chain sulfonated polyimide homopolymer NTDA-BSBSDA and preparation of a proton exchange membrane
[0036] Under the protection of nitrogen and mechanical stirring, add 2,2'-bis(3-sulfonic acid benzenesulfonyl)-4,4'-diaminobiphenyl (BSBSDA, 10mmol) into a 250mL dry three-necked flask 6.24g, 2.02-3.03g (20-30mmol) of triethylamine, and 45-90mL of m-cresol. Preferably, the optimal addition amounts in this embodiment are 2.42g (24mmol) and 60mL respectively. After completely dissolving, add 2.68g (NTDA, 10mmol) of 1,4,5,8-naphthalene tetracarboxylic dianhydride and 2.72-4.08g (20-30mmol) of phenylacetic acid, preferably, the optimal addition amount in this example It is 3.26g (22mmol). Under the protection of nitrogen, first raise the temperature of the reaction system to 50-120°C for 1-8h, and then continue to heat up to 150-200°C for 8-24h. Preferably, the two temperatures in this example are 80°C and 180°C respectively. ℃,...
Embodiment 2
[0038] Example 2: Synthesis of a sulfone group-bridged side-chain sulfonated polyimide copolymer NTDA-BSBSDA / ODA (1 / 1) and preparation of a proton exchange membrane
[0039] Under the protection of nitrogen and mechanical stirring, 3.12 g of 2,2'-bis(3-sulfobenzenesulfonyl)-4,4'-diaminobiphenyl (BSBSDA, 5mmol), triethylenediamine 1.12-1.68g (10-15mmol) and m-cresol 34-68mL, preferably, the optimal addition amount in this embodiment is 1.34g (12mmol) and 50mL. After the solid is completely dissolved, add 1.00 g of 4,4'-diaminodiphenyl ether (ODA, 5 mmol), 2.68 g of 1,4,5,8-naphthalene tetracarboxylic dianhydride (NTDA, 10 mmol) and 2.44-3.66 g of benzoic acid g (20-30mmol), preferably, the optimal addition amount in the present embodiment is 2.68g (22mmol). Under the protection of nitrogen, first raise the temperature of the reaction system to 50-120°C for 1-8h, then continue to heat up to 150-200°C for 8-24h, preferably, the optimum temperature points in this embodiment are 1...
Embodiment 3
[0041] Example 3: Synthesis of a sulfone group-bridged side-chain sulfonated polyimide copolymer NTDA-BSBSDA / ODA (2 / 1) and preparation of a proton exchange membrane
[0042] Under the protection of nitrogen and mechanical stirring, 2,2'-bis(3-sulfonic acid benzenesulfonyl)-4,4'-diaminobiphenyl 3.74g (BSBSDA, 6mmol), triethylamine 1.21-1.81g (12-18mmol) and m-cresol 34-68mL, preferably, the optimum addition amount of this embodiment is 1.41g (14mmol) and 45mL. After complete dissolution, add 0.60g of 4,4'-diaminodiphenyl ether (ODA, 3mmol), 2.41g of 1,4,5,8-naphthalene tetracarboxylic dianhydride (NTDA, 9mmol) and 2.20-3.29g of benzoic acid ( 18-27mmol), preferably, the optimum addition amount in the present embodiment is 2.44g (20mmol). Under the protection of nitrogen, first raise the temperature of the reaction system to 50-120°C for 1-8h, and then continue to heat up to 150-200°C for 8-24h. Preferably, the two temperatures in this example are 80°C and 180°C respectively. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com