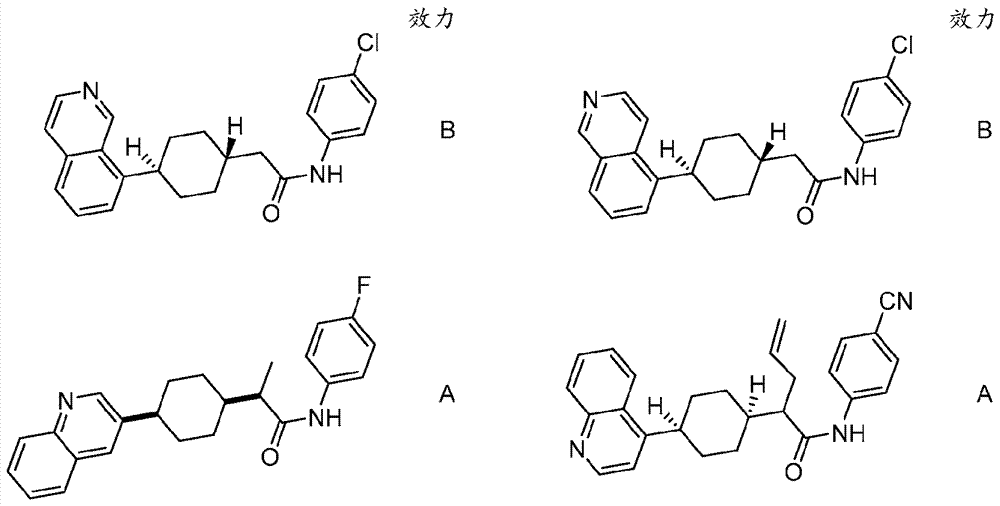
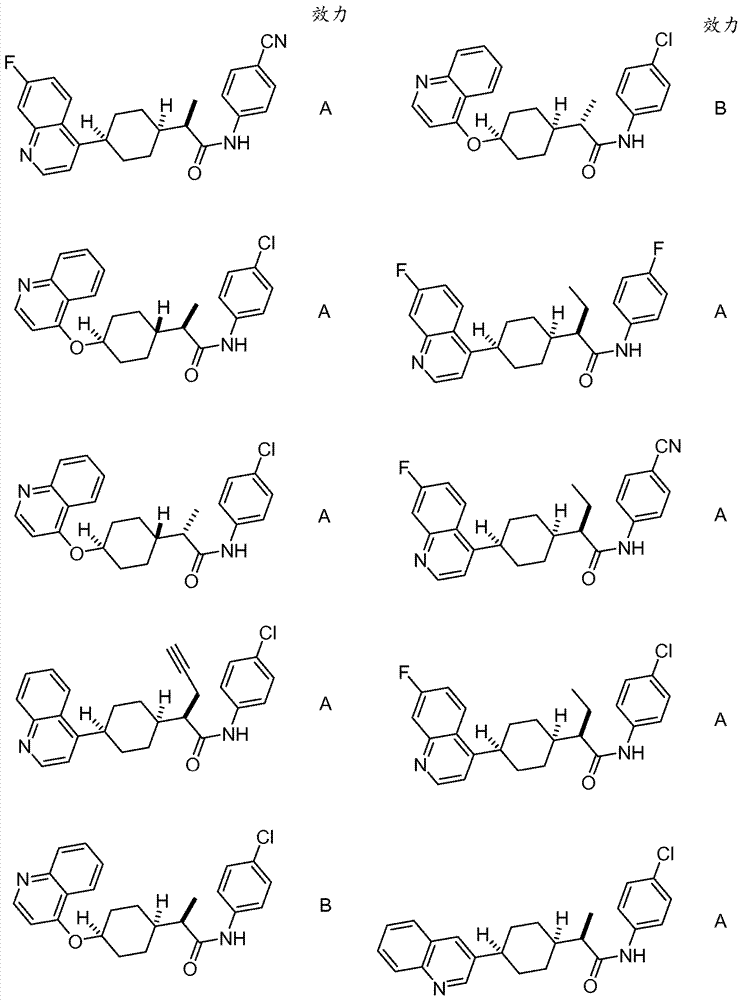
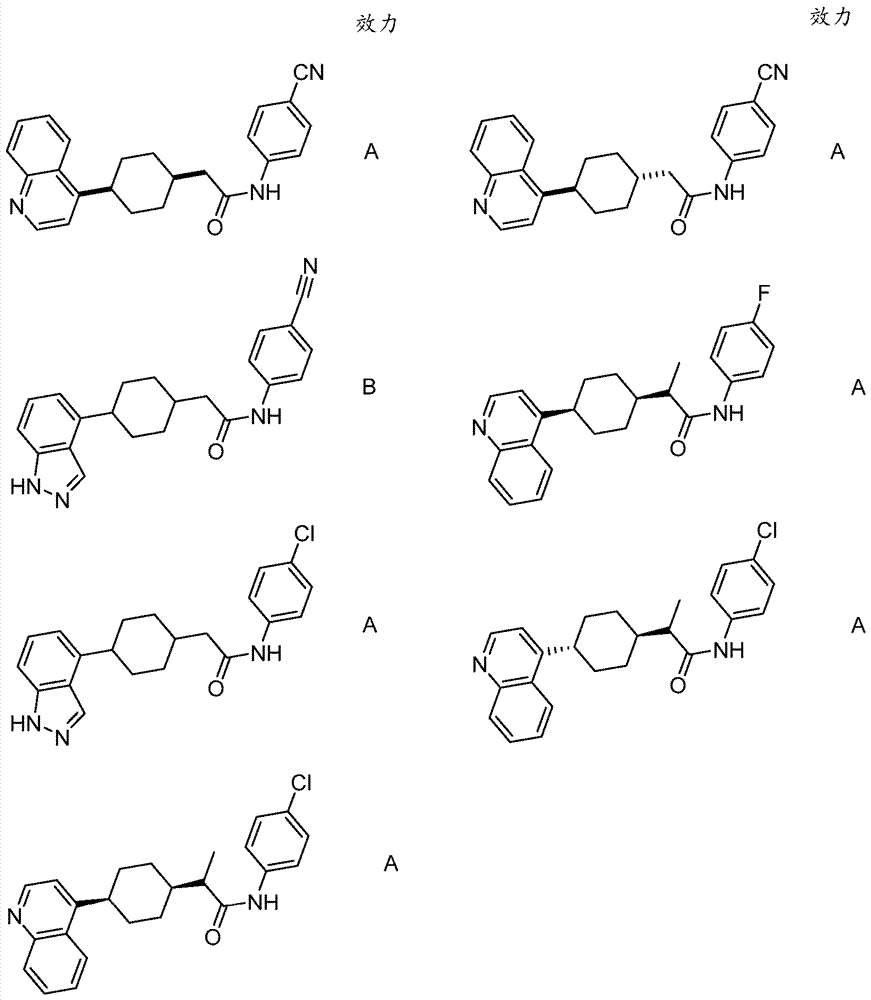
Immunoregulatory agents
A solvate and compound technology, which is applied in the field of compounds that regulate oxidoreductase indoleamine 2,3-dioxygenase, and can solve problems such as different functions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 and 2
[0341] N-(4-Chlorophenyl)-2-(trans-4-(quinolin-4-yl)cyclohexyl)acetamide
[0342]
[0343] N-(4-Chlorophenyl)-2-(4-(cis-quinolin-4-yl)cyclohexyl)acetamide
[0344]
[0345] Examples 1 and 2: N-(4-chlorophenyl)-2-(trans-4-(quinolin-4-yl)cyclohexyl)acetamide and N-(4-chlorophenyl)-2-( cis-4-(quinolin-4-yl)cyclohexyl)acetamide
[0346] Examples 1 and 2 were prepared using general procedures A, B and G. General procedure A used 7.91 g (25 mmol) ethyl 2-(4-(((trifluoromethyl)sulfonyl)oxy)cyclohex-3-en-1-yl)acetate and 4.56 g (26 mmol) quinoline -4-boronic acid. General procedure B used 20 wt% dry Pd / C (10 wt%) and methanol as solvent. General Procedure G used 100 mg ethyl 2-(4-(quinolin-4-yl)cyclohexyl)acetate (mixture of diastereomers) and 87 mg 4-chloroaniline. Purification using silica gel chromatography (0% to 60% ethyl acetate / toluene) afforded Example 1 (trans-diastereomer) as the first eluting isomer. trans-isomer 1 HNMR (400MHz; CDCl 3):δ8.85(d,J=4.6Hz,1H),8.0...
Embodiment 3 and 4
[0349] N-(4-cyanophenyl)-2-(trans-4-(quinolin-4-yl)cyclohexyl)acetamide hydrochloride
[0350]
[0351] N-(4-cyanophenyl)-2-(cis-4-(quinolin-4-yl)cyclohexyl)acetamide hydrochloride
[0352]
[0353] Examples 3 and 4: N-(4-cyanophenyl)-2-(trans-4-(quinolin-4-yl)cyclohexyl)acetamide hydrochloride and N-(4-cyanophenyl )-2-(cis-4-(quinolin-4-yl)cyclohexyl)acetamide hydrochloride
[0354] Examples 3 and 4 were prepared using general procedures A, B and G. General procedure A used 7.91 g (25 mmol) ethyl 2-(4-(((trifluoromethyl)sulfonyl)oxy)cyclohex-3-en-1-yl)acetate and 4.56 g (26 mmol) quinoline -4-boronic acid. General procedure B used 20 wt% dry Pd / C (10 wt%) and methanol as solvent. General Procedure G uses 100 mg ethyl 2-(4-(quinolin-4-yl)cyclohexyl)acetate (mixture of diastereomers) and 80 mg 4-cyanoaniline. Purification using silica gel chromatography (0% to 60% ethyl acetate / toluene) afforded Example 3 (trans-diastereomer) as the first eluting isomer. The free ba...
Embodiment 5 and 6
[0357] N-(4-fluorophenyl)-2-(trans-4-(quinolin-4-yl)cyclohexyl)acetamide
[0358]
[0359] N-(4-fluorophenyl)-2-(cis-4-(quinolin-4-yl)cyclohexyl)acetamide
[0360]
[0361] Examples 5 and 6: N-(4-fluorophenyl)-2-(trans-4-(quinolin-4-yl)cyclohexyl)acetamide and N-(4-fluorophenyl)-2-( cis-4-(quinolin-4-yl)cyclohexyl)acetamide
[0362] Prepared using general procedures A, B and G. General procedure A used 7.91 g (25 mmol) ethyl 2-(4-(((trifluoromethyl)sulfonyl)oxy)cyclohex-3-en-1-yl)acetate and 4.56 g (26 mmol) quinoline -4-boronic acid. General procedure B used 20 wt% dry Pd / C (10 wt%) and methanol as solvent. General Procedure G used 100 mg ethyl 2-(4-(quinolin-4-yl)cyclohexyl)acetate (mixture of diastereomers) and 76 mg 4-fluoroaniline. Purification using silica gel chromatography (0% to 60% ethyl acetate / toluene) afforded Example 5 (trans-diastereomer) as the first eluting isomer. trans-isomer 1 HNMR (400MHz; CDCl 3 ):δ8.85(d,J=4.5Hz,1H),8.05-8.15(m,2H),7.67-7.7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com