Application of molybdenum phosphide to catalytic hydrogen generation in alkaline formaldehyde solution
A molybdenum phosphide catalyst and formaldehyde solution technology, applied in the field of chemical applications, can solve the problems of limited resources, expensive precious metal catalysts and the like, and achieve the effect of increasing the rate of hydrogen production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
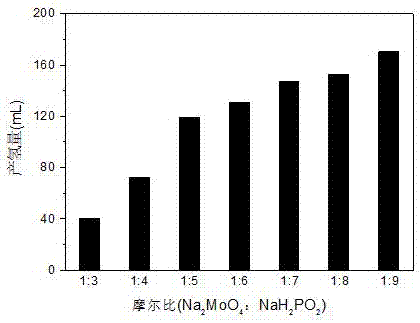
[0019] (1) Preparation of molybdenum phosphide catalyst. Weigh Na with a molar ratio of 1:3~1:9 2 MoO 4 and NaH 2 PO 2 (Na 2 MoO 4 The amount of the substance is 1 mmol), dissolved in 15 mL of water, stirred and dissolved, and dried at 60°C to obtain a solid reactant precursor. After grinding, the N 2 Calcined at 300 °C for 1 h in a tubular muffle furnace. Wash with deionized water and ethanol several times, and dry at 60°C to obtain molybdenum phosphide.
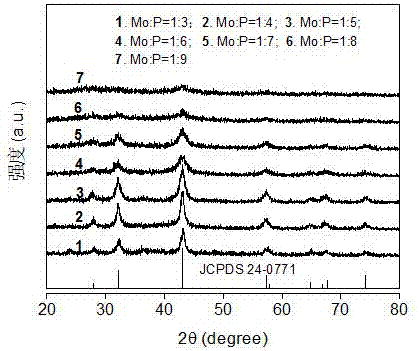
[0020] figure 1 Na in different molar ratios 2 MoO 4 and NaH 2 PO 2 X-ray diffraction pattern (XRD) of molybdenum phosphide prepared for the reactants. It can be seen from the figure that with NaH 2 PO 2 The increase of the amount of , the crystal form has not changed, all are MoP, but the diffraction peaks in the XRD pattern gradually become weaker.
[0021] (2) Evaluation of hydrogen production activity of molybdenum phosphide catalytic decomposition of formaldehyde solution. Molybdenum phosphide decompose...
Embodiment 2
[0024] (1) Preparation of molybdenum phosphide catalyst. Weigh 1 mmol Na 2 MoO 4 and 7 mmol NaH 2 PO 2 , dissolved in 15 ml of water, dissolved under stirring, and dried at 60°C to obtain a solid reactant precursor. After grinding in N 2 Calcined at 300 °C for 1 h. Wash with deionized water and ethanol several times, and dry at 60°C to obtain molybdenum phosphide.
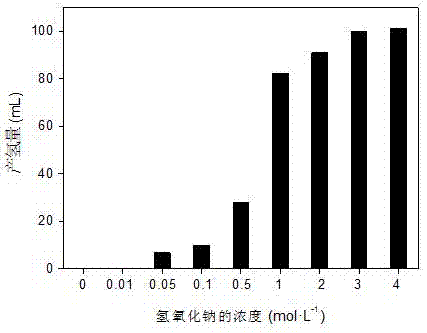
[0025] (2) The effect of sodium hydroxide concentration on the reaction activity of formaldehyde for hydrogen production. The evaluation of the hydrogen production reaction activity is the same as step (2) in Example 1. Weigh 20 mg of prepared molybdenum phosphide and add to 1 mol L -1 In the reaction solution of the formaldehyde solution, the concentration of sodium hydroxide in the solution is 0.01-4 mol L -1 range between. The reaction was stirred at room temperature (20°C) for 30 min. like image 3 As shown, at low alkali concentration (less than 0.5 mol . L -1 ), the hydrogen production activity ...
Embodiment 3
[0027] (1) The preparation of the catalyst is the same as Step 1 of Example 2.
[0028] (2) The effect of formaldehyde concentration on the reaction activity of formaldehyde hydrogen production. The evaluation of hydrogen production reaction activity is the same as step 2 in Example 1. Weigh 20 mg of prepared molybdenum phosphide and add to 1 mol L -1 In the reaction solution of sodium hydroxide solution, the concentration of formaldehyde in the reaction solution is 0.1-3 mol L -1 range between. The reaction was stirred at room temperature (20°C) for 30 min. like Figure 4 As shown, the hydrogen production activity increases with the increase of formaldehyde concentration, at a formaldehyde concentration of 2 mol L -1 to achieve the best. The optimized formaldehyde concentration is 1-3 mol L -1 between.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com