Method for catalyzing hydrogen production from formic acid
A formic acid and catalyst technology, which is applied in the field of hydrogen production from formic acid catalyzed by noble metal Pd-supported catalysts, can solve the problems of low hydrogen production activity and achieve high catalytic efficiency, good economic and environmental benefits, and improved dispersion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
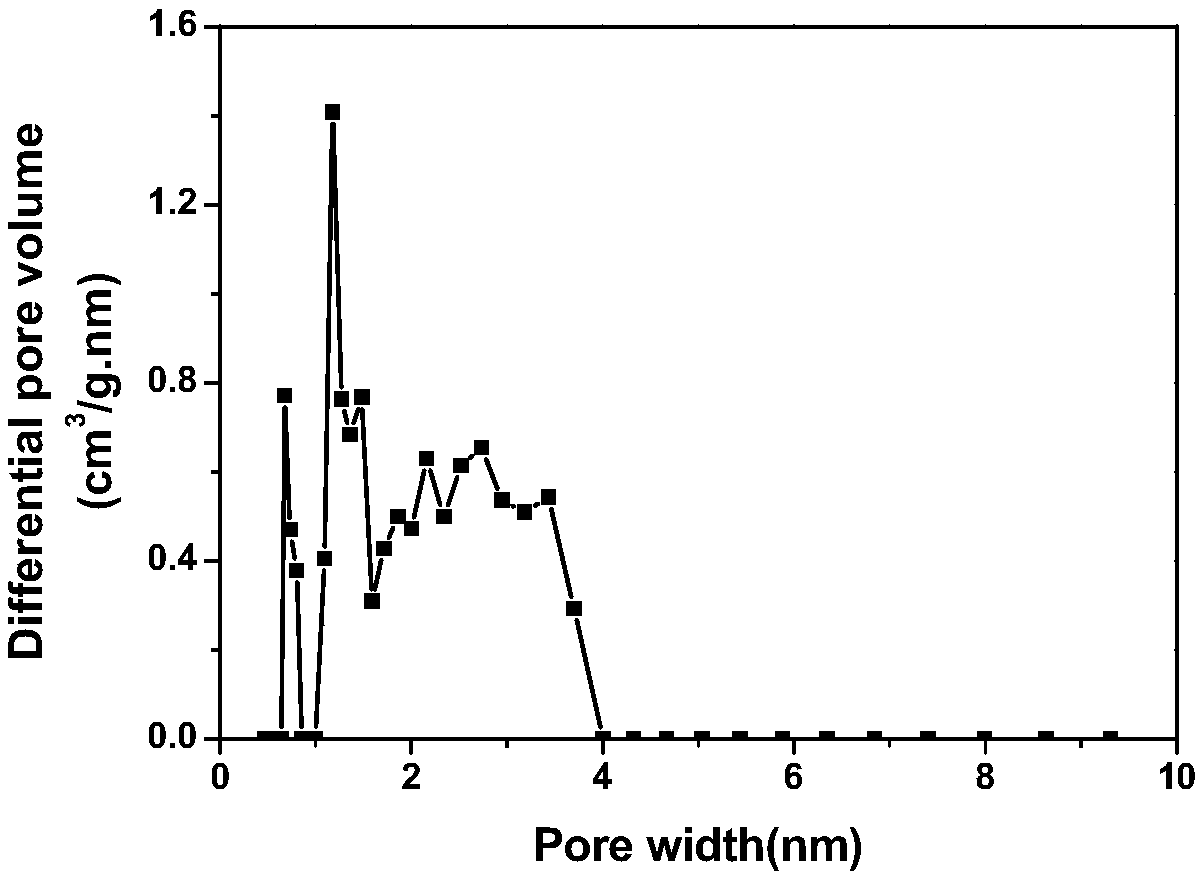
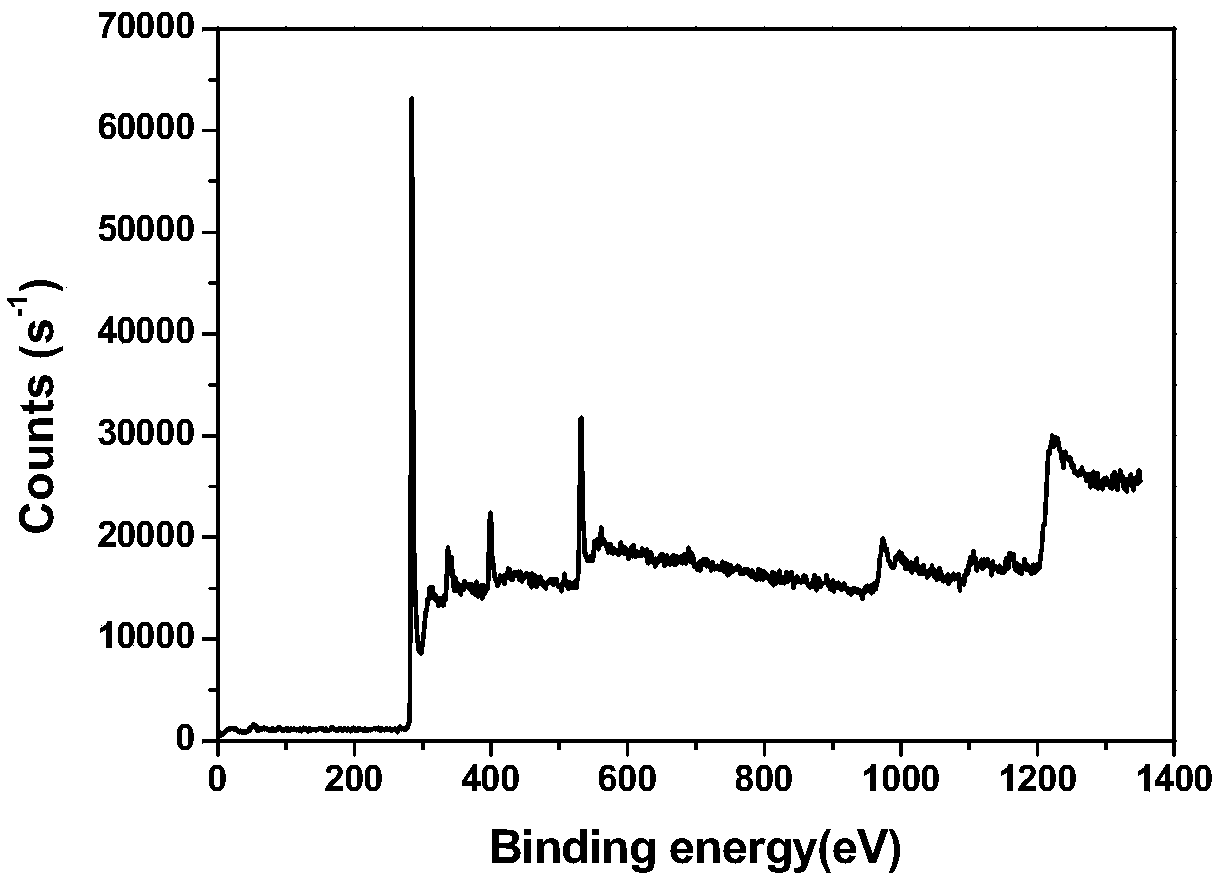
Image
Examples
Embodiment 1
[0033] Step 1: Synthesize CTF
[0034] 1) 1.596g of anhydrous zinc chloride (stored in a glove box) and 0.3g of 1,4-terephthalonitrile were ground in a glove box, and the resulting powder was transferred to a quartz tube;
[0035] 2) Use a vacuum pump to vacuum to a degree of 10 -2 Pa, after sealing, heat to 300°C for 60 hours, continue heating to 450°C for 5 hours;
[0036] 3) The obtained black block material is washed repeatedly with distilled water and 1.0M HCl solution after crushing, for removing residual zinc chloride;
[0037] 4) Wash the obtained black powder with distilled water and tetrahydrofuran, and dry it under vacuum at 150°C overnight to obtain CTF-450.
[0038] Step 2: Loading precious metal Pd
[0039] 1) Add 0.15g of CTF-450 to 10ml of deionized water, then add appropriate amount of PdCl 2 solution, stirred for 3 hours and then washed with 1.0M Na 2 CO 3Adjust the pH to 10.5 and continue stirring for 2 hours;
[0040] 2) Wash the material with deioni...
Embodiment 2
[0049] Step 1: Synthesize CTF
[0050] 1) 0.9576g of anhydrous zinc chloride (stored in a glove box) and 0.3g of 1,4-terephthalonitrile were ground in a glove box, and the resulting powder was transferred to a quartz tube;
[0051] 2) Use a vacuum pump to vacuum to a degree of 10 -2 Pa, after sealing, heat to 300°C for 60 hours, continue heating to 550°C for 6 hours;
[0052] 3) The obtained black block material is washed repeatedly with distilled water and 1.0M HCl solution after crushing, for removing residual zinc chloride;
[0053] 4) Wash the obtained black powder with distilled water and tetrahydrofuran, and dry it under vacuum at 150°C overnight to obtain CTF-550.
[0054] Step 2: Loading precious metal Pd
[0055] 1) Add 0.15g of CTF-550 to 10ml of deionized water, then add appropriate amount of PdCl 2 solution, after stirring for 3 hours, adjust the pH to 11, and continue stirring for 2 hours;
[0056] 2) Wash the material with deionized water until neutral, and ...
Embodiment 3
[0061] Step 1: Synthesize CTF
[0062] 1) 0.319g of anhydrous zinc chloride (stored in a glove box) and 0.3g of 1,4-terephthalonitrile were ground in a glove box, and the resulting powder was transferred to a quartz tube;
[0063] 2) Use a vacuum pump to vacuum to a degree of 10 -2 Pa, after sealing, heat to 300°C for 60 hours, continue heating to 650°C for 4 hours;
[0064] 3) The obtained black block material is washed repeatedly with distilled water and 1.0M HCl solution after crushing, for removing residual zinc chloride;
[0065] 4) Wash the obtained black powder with distilled water and tetrahydrofuran, and dry it under vacuum at 150°C overnight to obtain CTF-650.
[0066] Step 2: Loading precious metal Pd
[0067] 1) Add 0.15g of CTF-650 to 10ml of deionized water, then add appropriate amount of PdCl 2 solution, after stirring for 3 hours, adjust the pH to 9, and continue stirring for 2 hours;
[0068] 2) Wash the material with deionized water until neutral, and dr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com