Compounding method for N-ethyl carbazole
A synthesis method, ethyl carbazole technology, applied in the field of synthesis of N-ethyl carbazole, can solve problems such as the difficulty of vinyl hydrogenation and reduction, and achieve the effects of high product quality, cost reduction, and reduction of waste emissions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] In a 50ml glass pressure-resistant bottle, add 2g carbazole, 0.04g proton-removing agent KOH and 10ml N-methylpyrrolidone as a solvent, start stirring and raise the temperature to 80°C for a salt-forming reaction for 30 minutes to generate carbazole potassium salt, and then Pass through acetylene, react for 2.5 hours at 160°C under normal pressure, and obtain N-vinyl carbazole with a yield of 98.6%; in the next step, continue hydrogenation to generate N-ethyl carbazole, according to the catalyst and N-vinyl carbazole The molar concentration ratio of the azole substance is 1:2000, add Pd catalyst (support is MgO), pass 0.3MPaH 2 , hydrogenation reaction was carried out at 70°C for 3 hours to obtain N-ethylcarbazole with a yield of 98.8%.
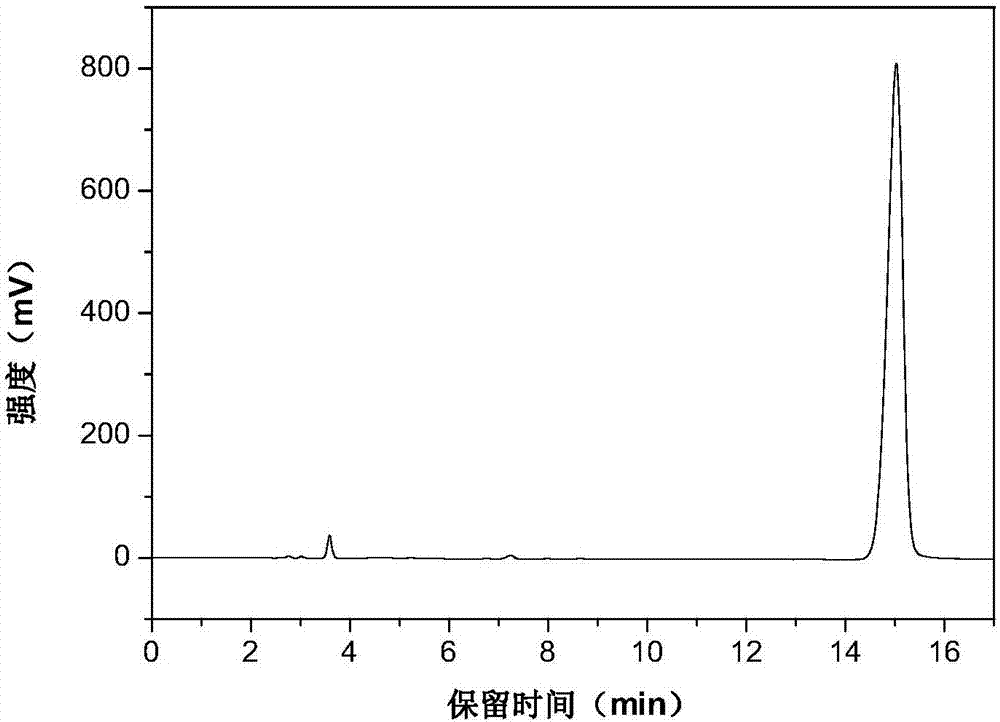
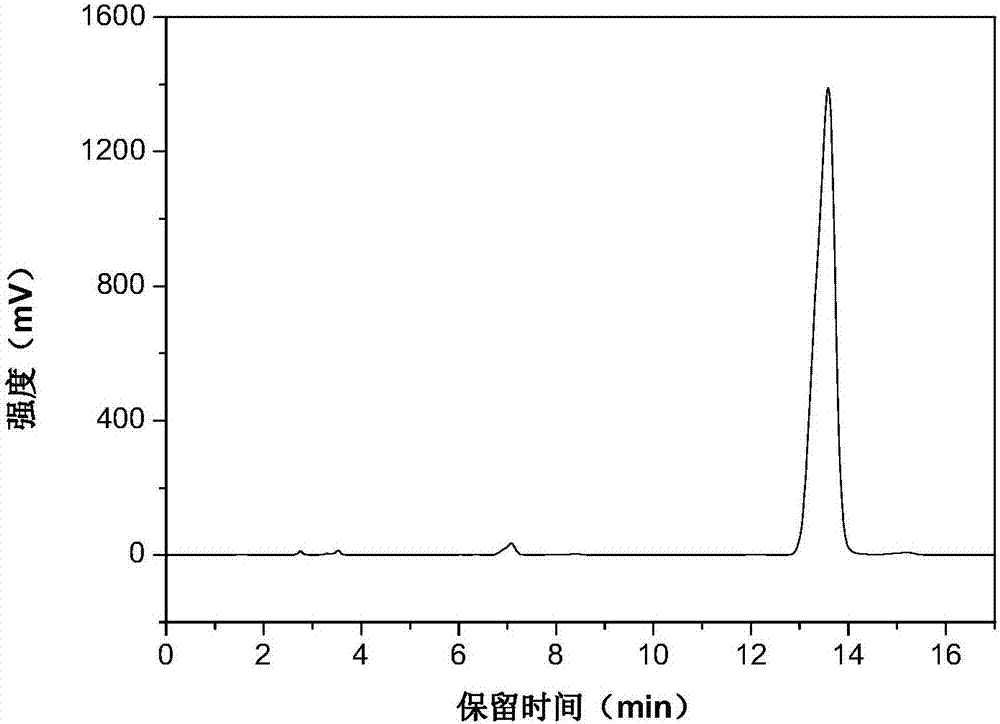
[0025] The high performance liquid phase chromatogram of the intermediate product N-vinylcarbazole of the embodiment of the present invention 1 sees figure 1 , the high performance liquid chromatogram of the product N-ethylcarbazole o...
Embodiment 2
[0027] In a 50ml glass pressure-resistant bottle, add 2g carbazole, 0.08g proton-removing agent KOH and 10ml N-methylpyrrolidone as a solvent, start stirring and heat up to 80°C for a salt-forming reaction for 20 minutes to generate carbazole potassium salt, and then Pass through acetylene, react at 160°C and normal pressure for 5 hours, and obtain N-vinylcarbazole with a yield of 99.0%; in the next step, continue hydrogenation to generate N-ethylcarbazole, according to the catalyst and N-vinylcarbazole The molar concentration ratio of the substance is 1:2000, add Pd catalyst (support is MgO), pass 0.3MPaH 2 , hydrogenation reaction was carried out at 70°C for 3 hours to obtain N-ethylcarbazole with a yield of 98.7%.
Embodiment 3
[0029] In a 50ml glass pressure-resistant bottle, add 2g carbazole, 0.12g proton-removing agent KOH and 10ml N-methylpyrrolidone as a solvent, start stirring and raise the temperature to 80°C for a salt-forming reaction for 20 minutes to generate carbazole potassium salt, and then Pass through acetylene, react for 2.5 hours at 160°C under normal pressure, and obtain N-vinyl carbazole with a yield of 98.9%. The molar concentration ratio of the azole substance is 1:2000, add Pd catalyst (support is MgO), pass 0.3MPaH 2 , hydrogenation reaction was carried out at 70°C for 3 hours to obtain N-ethylcarbazole with a yield of 98.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com