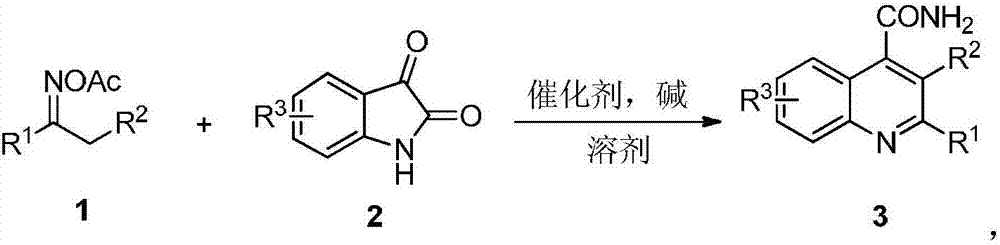
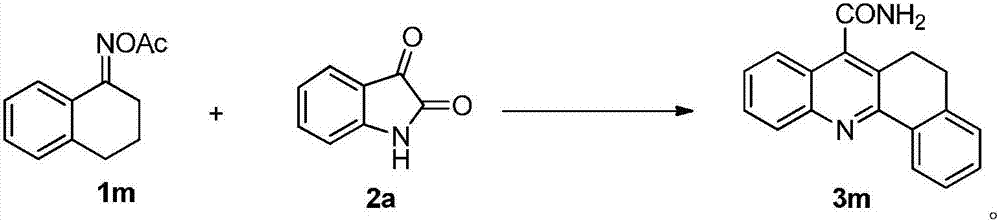
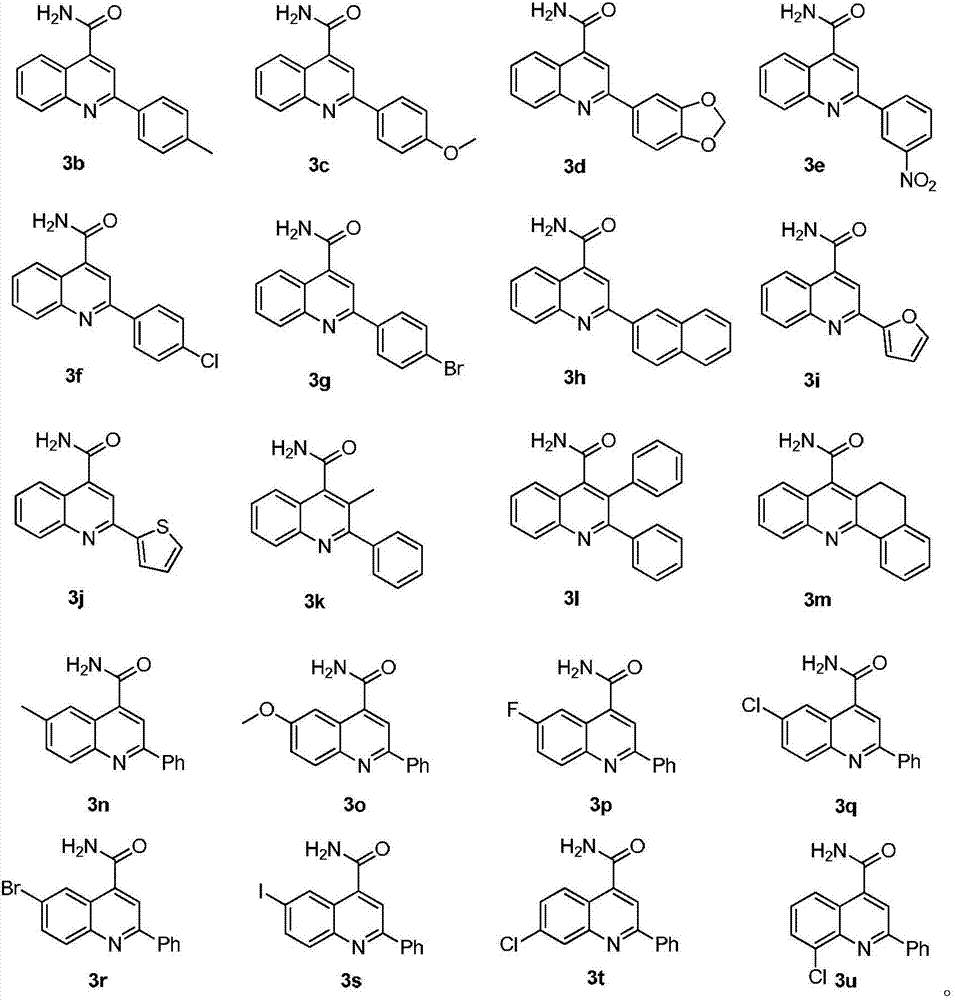
Synthetic method of quinoline-4-formamides compound
A technology of formamides and synthesis methods, which is applied in the field of synthesis of quinoline-4-carboxamides, can solve problems such as application limitations, and achieve the effects of simple operation, simple process, and avoiding environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016]
[0017] Add 1a (0.5mmol, 88.6mg), 2a (0.5mmol, 73.6mg), iodine (0.25mmol, 63.5mg), triethylamine (0.25mmol, 25.3mg) and chlorobenzene (2mL) into a 25mL sealed tube , and then placed in an oil bath at 130 °C and stirred for 10 h. Add 50mL of water to quench the reaction, extract with ethyl acetate (50mL×3), and then the organic phase is treated with 10% Na 2 S 2 o 3 The solution was washed successively with saturated brine, and dried over anhydrous sodium sulfate. Filtration, spin-drying, and silica gel column separation (petroleum ether / ethyl acetate=5 / 1) gave white solid product 2-phenylquinoline-4-carboxamide 3a (106.7 mg, 86%). The characterization data of this compound are as follows: 1 H NMR (600MHz, DMSO-d 6 ):δ(ppm)8.36(s,1H),8.35–8.30(m,2H),8.29(d,J=7.8Hz,1H),8.18(s,1H),8.14(d,J=7.8Hz, 1H),7.99(s,1H),7.85–7.79(m,1H),7.68–7.62(m,1H),7.62–7.55(m,2H),7.55–7.50(m,1H); 13 C NMR (150MHz, DMSO-d 6 )δ(ppm)168.7,155.8,148.0,143.1,138.3,130.1,129.9,129.5,128.9...
Embodiment 2
[0019] Add 1a (0.5mmol, 88.6mg), 2a (0.5mmol, 73.6mg), iodine (0.25mmol, 63.5mg), triethylamine (0.25mmol, 25.3mg) and chlorobenzene (2mL) into a 25mL sealed tube , and then placed in an oil bath at 120°C and stirred for 10 h. Add 50mL of water to quench the reaction, extract with ethyl acetate (50mL×3), and then the organic phase is treated with 10% Na 2 S 2 o 3 The solution was washed successively with saturated brine, and dried over anhydrous sodium sulfate. Filtered, spin-dried, separated by silica gel column (petroleum ether / ethyl acetate=5 / 1) to obtain the target product 3a (104.2 mg, 84%).
Embodiment 3
[0021] Add 1a (0.5mmol, 88.6mg), 2a (0.5mmol, 73.6mg), iodine (0.25mmol, 63.5mg), diethylamine (0.25mmol, 18.3mg) and chlorobenzene (2mL) into a 25mL sealed tube , and then placed in an oil bath at 120°C and stirred for 10 h. Add 50mL of water to quench the reaction, extract with ethyl acetate (50mL×3), and then the organic phase is treated with 10% Na 2 S 2 o 3 The solution was washed successively with saturated brine, and dried over anhydrous sodium sulfate. Filtered, spin-dried, separated by silica gel column (petroleum ether / ethyl acetate=5 / 1) to obtain the target product 3a (83.1 mg, 67%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com