Novel synthesis method for phosphate compounds
A technology of phosphate esters and compounds, applied in the field of chemical synthesis, to achieve the effect of large-scale production, cheap raw materials, and easy large-scale production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
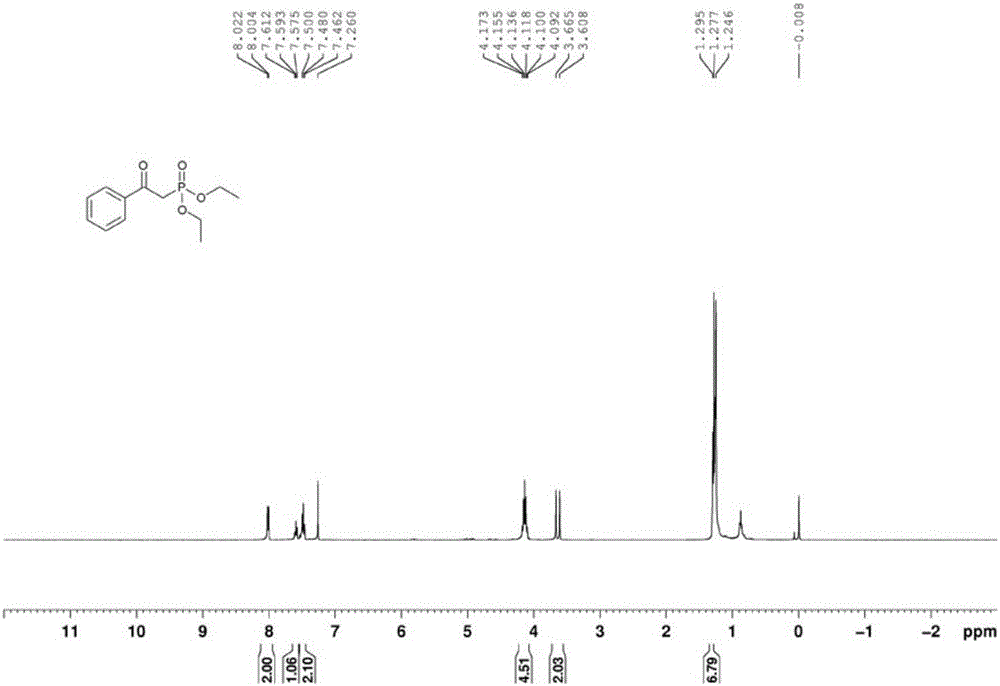
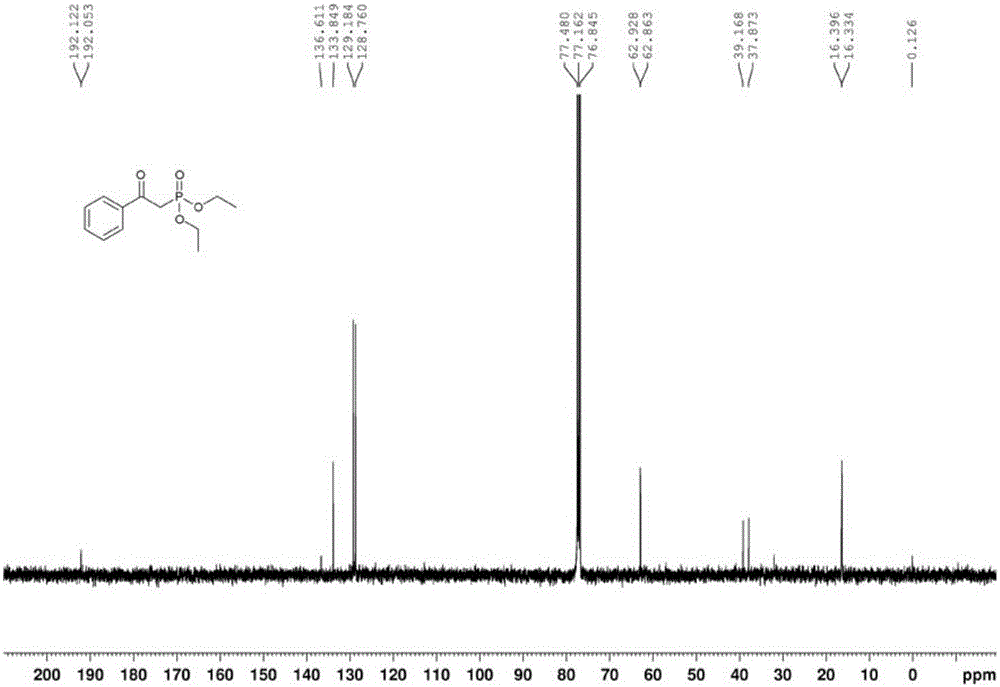
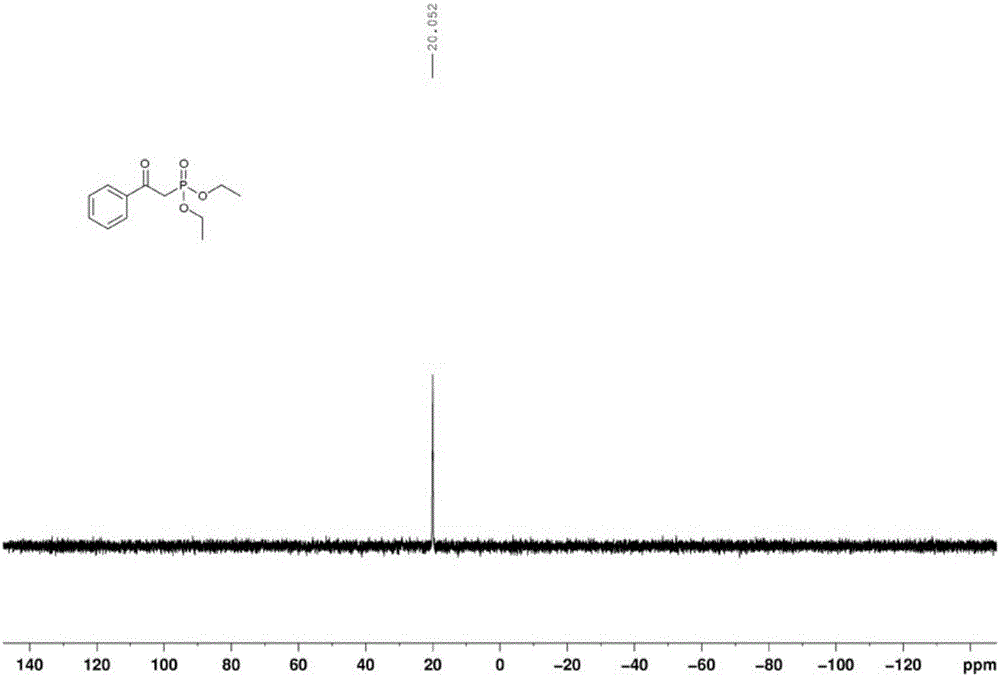
[0035] In a clean and dry 50ml Schlenk reaction tube, sequentially add 2mmol of 1-phenylethanol, 1mmol of diethyl phosphite, 0.2mmol of cobalt acetate, and 0.2mmol of N-hydroxyphthalimide, and then in the above mixture 5ml of toluene was added as a solvent, the reaction tube was fed with 1 atmosphere of oxygen, the reaction tube was sealed, and the reaction tube was placed in an oil bath at 80° C. for 12 hours. After the reaction, the reaction mixture was directly spin-dried, and the residue obtained was separated by column separation with petroleum ether and ethyl acetate (volume ratio: 2:1). , Phosphorus spectrum analysis, confirmed that the target product was α-diethyl phosphate acetophenone, and the yield was 84%.
[0036] The proton nuclear magnetic resonance spectrum of the product prepared in this embodiment is as follows: Figure 1a As shown, the carbon NMR spectrum is as Figure 1b As shown, the NMR phosphorus spectrum is as Figure 1c shown.
Embodiment 2
[0038] In a clean and dry 50ml Schlenk reaction tube, sequentially add 1mmol of 1-phenylethanol, 1mmol of diethyl phosphite, 0.1mmol of cobalt acetylacetonate, and 0.1mmol of N-hydroxyphthalimide, and then add 6ml of N,N-dimethylformamide was added as a solvent, the reaction tube was fed with 1 atmosphere of oxygen, the reaction tube was sealed, and the reaction tube was placed in an oil bath at 90°C for 18 hours. After the reaction, the reaction mixture was directly spin-dried, and the residue obtained was separated by column separation with petroleum ether and ethyl acetate (volume ratio: 2:1). , Phosphorus spectrum analysis, confirmed that the target product was α-diethyl phosphate acetophenone, and the yield was 75%.
[0039] The proton nuclear magnetic resonance spectrum of the product prepared in this embodiment is as follows: Figure 1a As shown, the carbon NMR spectrum is as Figure 1b As shown, the NMR phosphorus spectrum is as Figure 1c shown.
Embodiment 3
[0041] In a clean and dry 50ml Schlenk reaction tube, sequentially add 2mmol of 1-(4-bromophenyl)-ethanol, 1mmol of diethyl phosphite, 0.2mmol of cobalt acetate, and 0.1mmol of N-hydroxyphthalimide , and then add 6ml of N,N-dimethylformamide as a solvent to the above mixture, inject 1 atmosphere of oxygen into the reaction tube, seal the reaction tube, and place it in an oil bath at 90°C for 15 hours to react. After the reaction, the reaction mixture was directly spin-dried, and the residue obtained was separated by column separation with petroleum ether and ethyl acetate (volume ratio: 2:1). , Phosphorus spectrum analysis, confirmed that the target product was α-diethyl phosphate-4-bromoacetophenone, and the yield was 72%.
[0042] The proton nuclear magnetic resonance spectrum of the product prepared in this embodiment is as follows: Figure 2a As shown, the carbon NMR spectrum is as Figure 2b As shown, the NMR phosphorus spectrum is as Figure 2c shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com