Anti-interleukin 17A antibody and preparation method and application thereof
An antibody and chimeric antibody technology, applied in the field of medicine, can solve problems such as unclear downstream signals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0136] Antibody preparation
[0137] Any method suitable for producing monoclonal antibodies can be used to produce anti-IL-17A antibodies of the invention. For example, animals can be immunized with linked or naturally occurring IL-17A homodimers or fragments thereof. Suitable immunization methods may be used, including adjuvants, immunostimulants, repeated booster immunizations, one or more routes may be used.
[0138]Any suitable form of IL-17 can be used as an immunogen (antigen) for producing non-human antibodies specific to IL-17A and screening the biological activity of the antibodies. The stimulating immunogen can be full-length mature human IL-17A, including natural homodimers, or single / multiple epitope-containing peptides. Immunogens can be used alone or in combination with one or more immunogenicity enhancers known in the art. Immunogens can be purified from natural sources, or produced in genetically modified cells. The DNA encoding the immunogen can be genomi...
Embodiment 1
[0169] Example 1 Anti-human IL-17A mouse monoclonal antibody—preparation method of original antibody
[0170] 1.1 Preparation of hybridoma cells producing mouse monoclonal antibodies
[0171] Firstly, human IL-17A protein was used as antigen, emulsified with adjuvant, and multi-point subcutaneously immunized BALB / c mice to monitor the serum titer of immunized mice. After reaching the requirements, the spleen cells and myeloma (Sp2 / c) mice were collected. 0) The cells are fused and screened by HAT to obtain hybridoma polyclonal cells.
[0172] 1.2 Indirect ELISA—the screening method of hybridoma cells
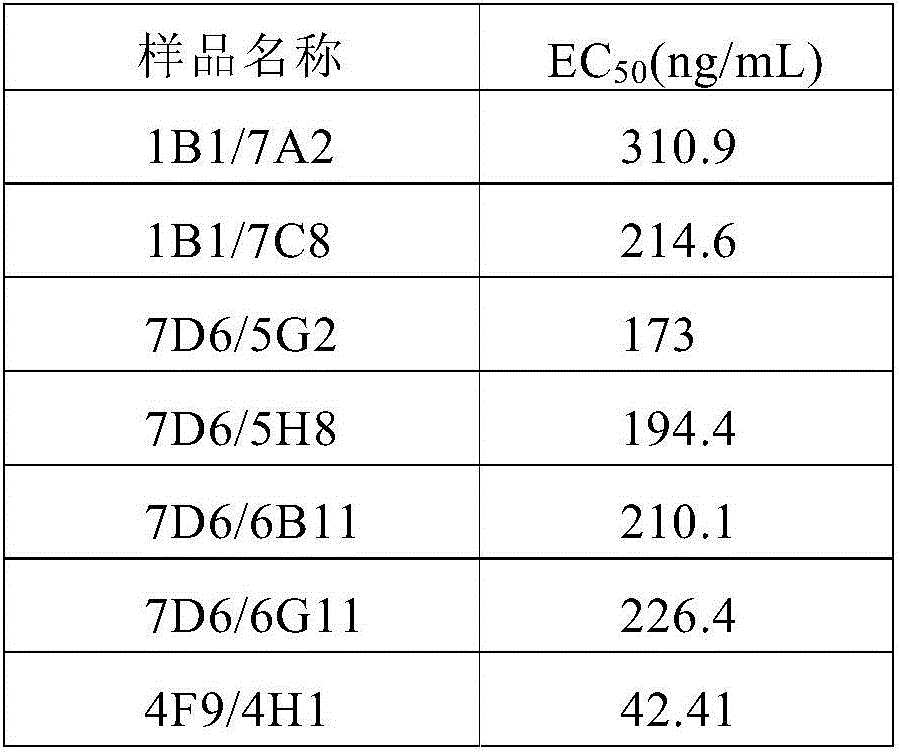
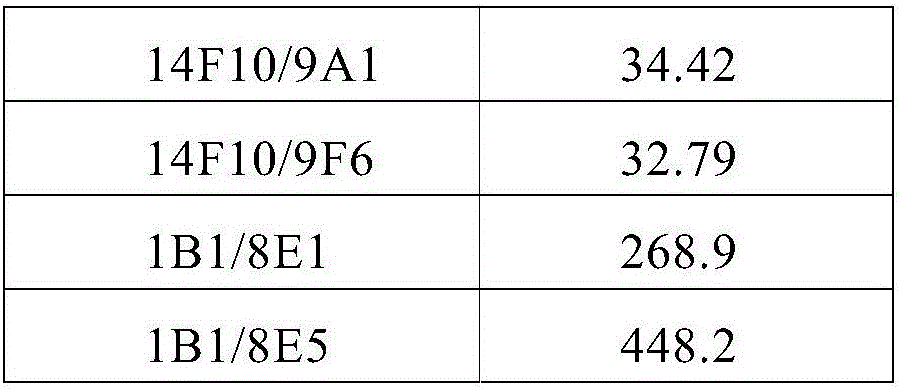
[0173] Use the ELISA detection method to screen out polyclonals with high specific binding, carry out monoclonal culture, and then use the ELISA method to screen the monoclonal cell lines with high specific binding; through the HT1080 cells to IL-6 release test, screen the cells with functional The effect of monoclonal cell lines, and then use the Biacore method to analyze the...
Embodiment 2
[0183] Example 2 Anti-IL-17A antibody V-gene sequence cloning
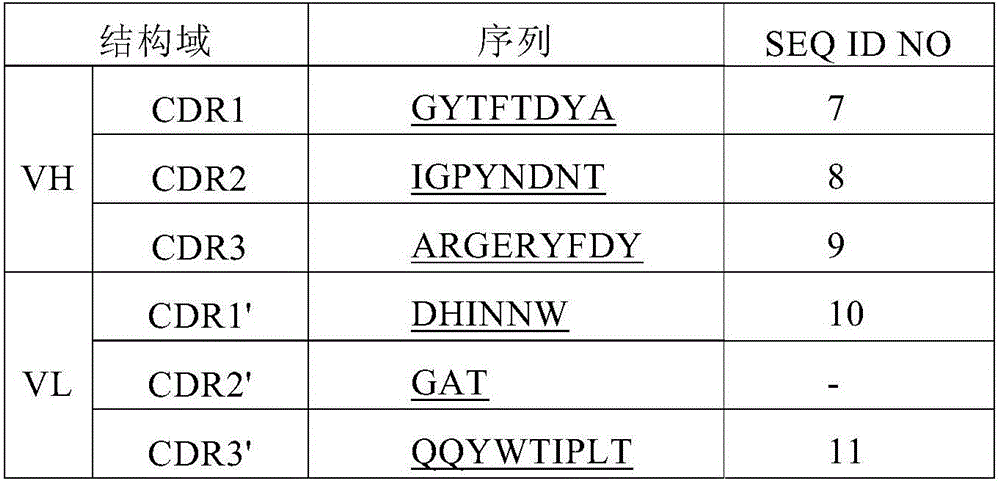
[0184] Based on the 5' RACE technique, the DNA sequence encoding the variable region of the mouse antibody expressed by the hybridoma 14F10 / 9F6 was determined. Briefly, heavy and light chain gene-specific cDNAs were prepared using SMART 5'RACE Synthesis Kit (TAKARA, No. 634859) according to the manufacturer's instructions. PCR products were analyzed by agarose gel electrophoresis. The variable regions of both the heavy and light chains are approximately 500 base pairs in size. The amplified PCR product with the appropriate band size obtained from the reaction was cloned into the vector pEASY-BluntSimple plasmid (Beijing Quanshijin, No.CB111-02), and transformed into Stellar Escherichia coli competent cells (TAKARA, No.636763) middle. Clones were screened by colony PCR with universal M13 forward or reverse primers, and 2-3 clones from each reaction were selected for DNA sequencing analysis. The results of each ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com