Heterocyclic compound and organic solar cell comprising same
一种杂环化合物、太阳能电池的技术,应用在硅有机化合物、有机化学、有机染料等方向,能够解决提高制造成本等问题,达到提高溶解度、抑制供体尺寸的增加、开路电压和短路电流增加的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
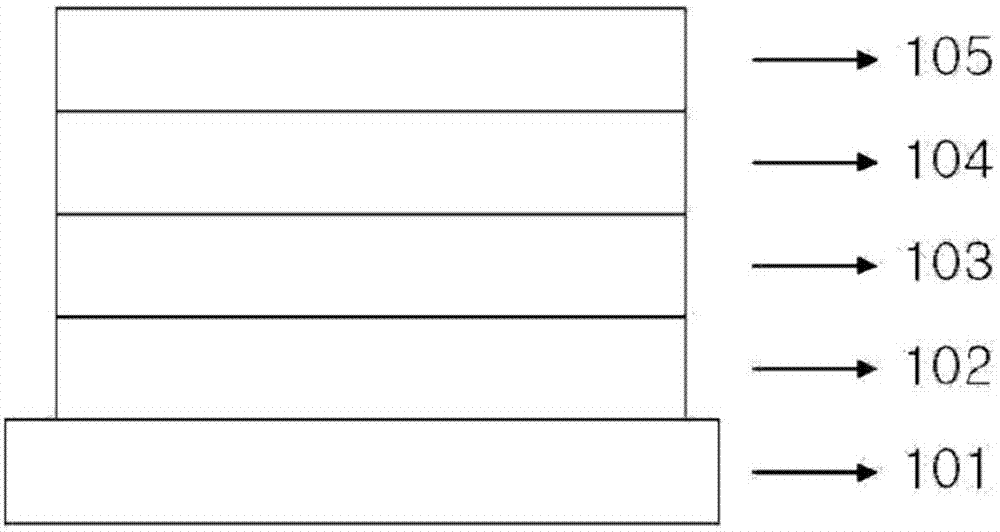
Image
Examples
Embodiment approach
[0126] According to an exemplary embodiment of the present specification, R9 is methyl.
[0127] According to an exemplary embodiment of the present specification, R10 is methyl.
[0128] According to an exemplary embodiment of the present specification, R11 is methyl.
[0129] According to an exemplary embodiment of the present specification, R12 is methyl.
[0130] According to an exemplary embodiment of the present specification, R13 is methyl.
[0131] According to an exemplary embodiment of the present specification, R14 is methyl.
[0132] According to an exemplary embodiment of the present specification, R15 is methyl.
[0133] According to an exemplary embodiment of the present specification, in Chemical Formula 2, R16 and R17 are hydrogen.
[0134] According to an exemplary embodiment of the present specification, [Push] comprises one or two or more of the following: substituted or unsubstituted arylene, and a group comprising N, O, S, Si and Ge A substituted or ...
preparation example 1
[0245] Preparation Example 1. Preparation of Compound 1
[0246] (1) Preparation of compound 1-b
[0247]
[0248] After dissolving 1-a (5.29 g, 6 mmol) in 150 mL of chloroform, N-bromosuccinimide (1.28 g, 7.2 mmol) was injected thereto at room temperature, and the resulting mixture was stirred for 48 hours. After the reaction, the reactant was added to 250 mL of water, and extracted with dichloromethane. After this time, the remaining water was removed over magnesium sulfate, and the solvent was removed under reduced pressure. The remaining product was purified with a silica gel column (eluent: hexane:dichloromethane=2:1) to obtain black powder 1-b. (yield: 71%)
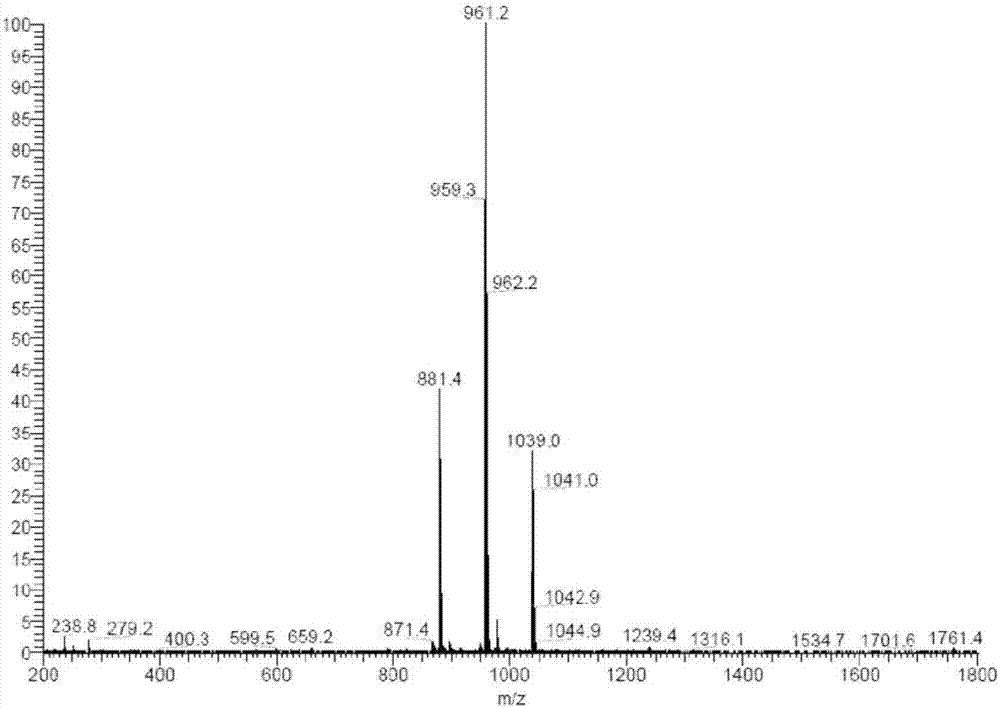
[0249] figure 2 is a graph showing the MS spectrum of compound 1-b.
[0250] (2) Preparation of compound 1-c
[0251]
[0252] 1-b (4.092 g, 4.26 mmol) and 2-aldehyde-thiophene borate (0.935 g, 6 mmol) were dissolved in 200 mL of tetrahydrofuran and 50 mL of 2M potassium carbonate, to which was added ...
preparation example 2
[0270] Preparation Example 2. Preparation of Compound 2
[0271]
[0272] Prepared in the same manner as the preparation of Compound 1 above, except that 40 mL of CHCl was used in the preparation of Compound 1 3 Compound B (1.09 g, 0.4 mmol) and DiCN-rhodanine (0.773 g, 3 mmol) in . (yield: 71%)
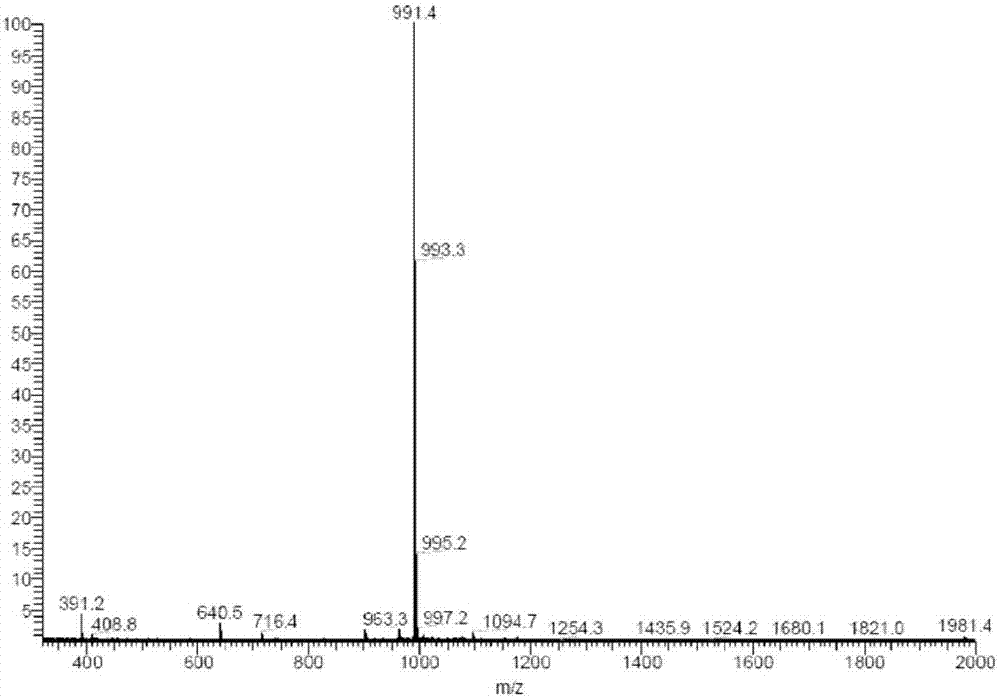
[0273] Figure 11 is a graph showing the MS spectrum of compound 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com