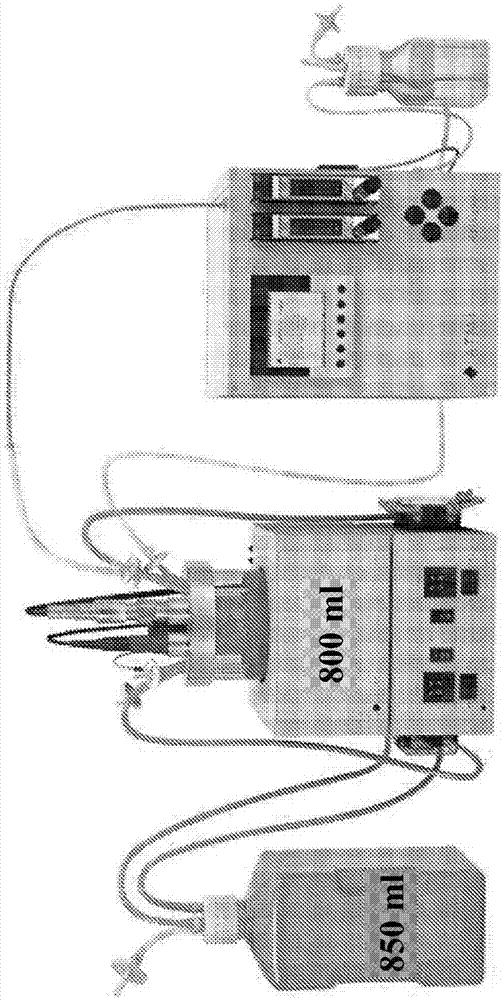
Methods for producing virus for vaccine production
A virus and enterovirus technology, applied in the field of virus production for vaccine preparation, can solve the problem of not being well suited for culturing animal cells and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0286] Example 1: Evaluation of cell growth in iCELLis NANO
[0287] Evaluation of Vero cell line growth in iCELLis NANO bioreactors.
[0288] method
[0289] Cell and Nuclei Counts
[0290] For iCELLis NANO, cells are counted using the nuclei counting method, while for Cell-Stacks, cells are counted using the trypan blue method.
[0291] Glucose / Lactate Measurement
[0292] Lactate was measured using a ScoutTM milk hydrometer. Glucose was measured using the ACCU-CHEK Aviva Plus system.
[0293] Vero Recovery
[0294] Perform Vero cell recovery. A 1-ml vial of Vero cells was thawed quickly (less than 3 minutes), swabbed with IsaSept, and mixed with 19 mL of pre-warmed DMEM D1145 supplemented with 10% FBS and 2 mM glutamine in a T-75 flask. An aliquot (0.4 ml) was removed for cell count and cell viability assay and the T-75 flask was placed in a CO2 (5%) incubator at 37°C. The next day, the spent medium was discarded and replaced with fresh medium (20 ml) and the cult...
Embodiment 2
[0314] Example 2: At 0.52E6 cells / cm in iCELLis NANO 2 Evaluation of EV71 Infection at Cell Densities
[0315] The kinetics and yield of EV71 virus produced by Vero cells grown in iCELLis NANO were evaluated. The 1X bioreactor was compacted using a 40ml fixed bed and EV71 infection was performed at a cell density of 0.52E6 cells / cm2.
[0316] method
[0317] NANO was inoculated as described in Example 1 and a total culture volume of 1,650 mL was used. The medium was completely replaced on day 3. On day 6, the cell density reached 0.52E6 cells / cm. A previously characterized Vero-adapted EV71 strain was used. Amino acid mutations in Vero-adapted EV71 strains at passage 8 (P8) are shown in Table 2.
[0318] Table 2: Amino acid mutations in Vero-adapted EV71.
[0319]
[0320] The bioreactor was rinsed twice with DPBS and infection with EV71 was started as described in Example 1 using DMEM D1145 supplemented with 2 mM glutamine but without FBS. Given the fact that the...
Embodiment 3
[0334] Example 3: In iCELLis NANO at 0.25E6 cells / cm 2 Evaluation of EV71 Infection at Cell Densities
[0335] The kinetics and yield of EV71 virus produced by Vero cells grown in iCELLis NANO were evaluated. A 1.5X bioreactor was compacted using a 40ml fixed bed and EV71 infection was performed at a cell density of 0.25E6 cells / cm2.
[0336] method
[0337]NANO was inoculated as described in Example 1 and the total culture volume used was 2,500 mL. No media replacement was performed during the pre-culture phase. On day 3, the cell density reached 0.25E6 cells / cm2. The bioreactor was rinsed with DPBS and infection with EV71 was started using 0.024 MOI. The infection medium used was the same as described but without FBS, as described in Example 2. The culture volume used during infection was 2,500 mL (ie volume / surface ratio 0.31 mL / cm2). As a control, Cell-Stack 5 (CS-5) was cultured and infected as described in Example 2. The composition of the medium used for Vero ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| porosity | aaaaa | aaaaa |
| porosity | aaaaa | aaaaa |
| porosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com