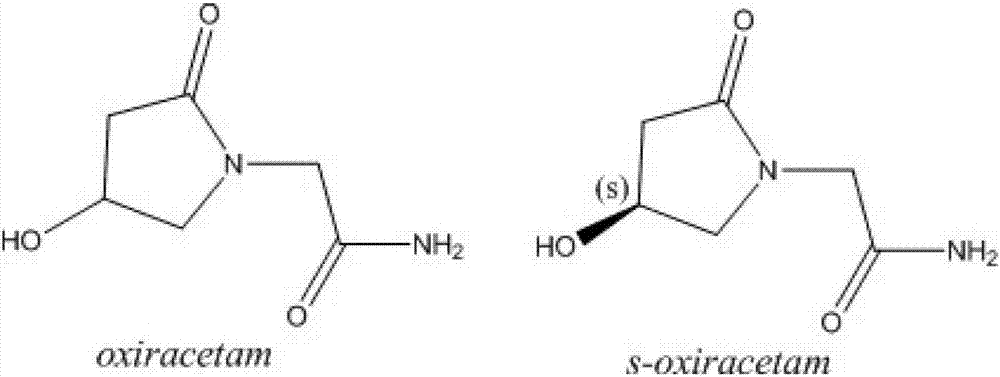
Levo-oxiracetam freeze-dried powder for injection
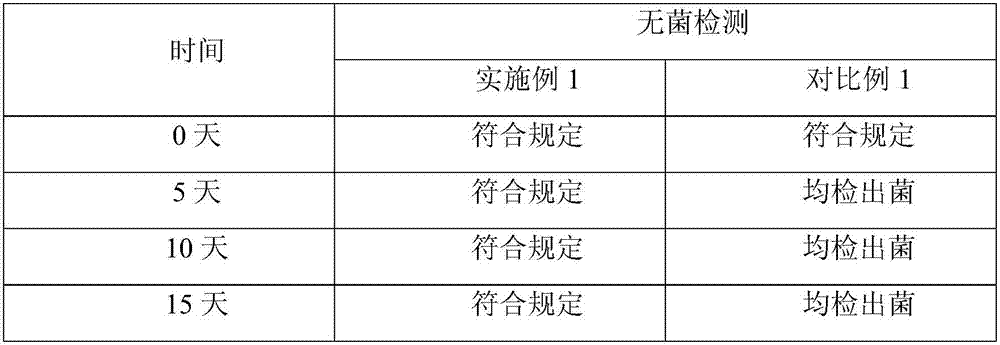
A technology for freeze-dried powder and injection, which is applied in the field of freeze-dried powder of Levo-Oxiracetam for injection, can solve the problems of low sublimation temperature, content drop, scattering, etc., achieve qualified sterility inspection, shorten production cycle, and improve sublimation. effect of temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
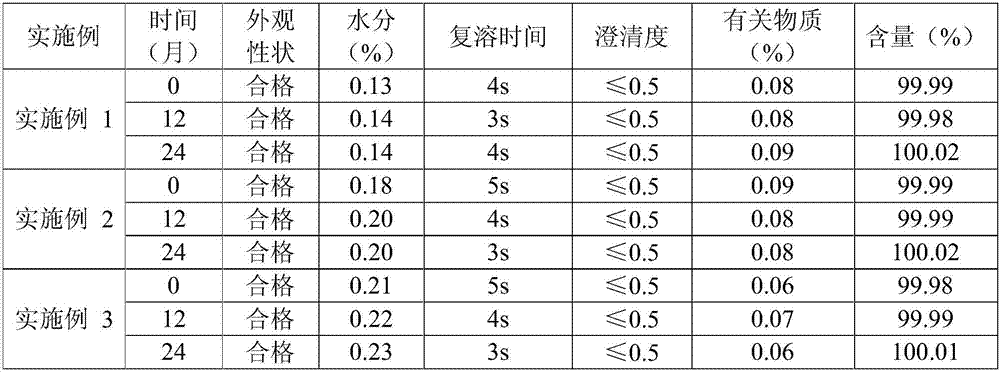
Embodiment 1
[0017] The prescription of the L-Oxiracetam lyophilized powder for injection of Example 1 is shown in the following table:
[0018] prescription
Weight percentage
L-Oxiracetam
200g
100g
140g
60g
Tween 80
2g
4g
Water for Injection
Add to 1000mL
[0019] The preparation method of L-Oxiracetam lyophilized powder for injection of Example 1 includes the following steps:
[0020] Dissolve the prescription amount of L-Oxiracetam, methionine, lactose, triethanolamine, Tween 80 and phenol in water for injection, adjust the pH to 5.5 with 0.1 mol / L hydrochloric acid solution, and then freeze dry according to the following steps :
[0021] (1) Pre-freezing stage: freeze the temperature to -15°C within 10 minutes; then increase the temperature to -10°C with a heating time of 40 minutes; again freeze the temperature to -35°C within 10 minutes; then increase the temperature to -25°C and increase the temperature The time is 100min; finally t...
Embodiment 2
[0025] The prescription of the lyophilized L-Oxiracetam powder for injection of Example 2 is shown in the following table:
[0026] prescription
Weight percentage
L-Oxiracetam
200g
80g
lactose
100g
40g
Tween 80
3g
phenol
2g
Water for Injection
Add to 1000mL
[0027] The preparation method of L-Oxiracetam lyophilized powder for injection of Example 2 includes the following steps:
[0028] Dissolve the prescription amount of L-Oxiracetam, methionine, lactose, triethanolamine, Tween 80 and phenol in water for injection, adjust the pH to 5.5 with 0.1 mol / L hydrochloric acid solution, and then freeze-dry according to the following steps :
[0029] (1) Pre-freezing stage: freeze the temperature to -15°C within 5 minutes; then increase the temperature to -9°C, and the heating time is 30 minutes; again freeze the temperature to -32°C within 5 minutes; then increase the temperature to -20°C and increase the temperature The time is 90min; finally the ...
Embodiment 3
[0033] The prescription of Levoxiracetam lyophilized powder for injection in Example 3 is shown in the following table:
[0034] prescription
Weight percentage
L-Oxiracetam
200g
120g
lactose
180g
80g
Tween 80
4g
phenol
6g
Water for Injection
Add to 1000mL
[0035] The preparation method of L-Oxiracetam lyophilized powder for injection of Example 3 includes the following steps:
[0036] Dissolve the prescription amount of L-Oxiracetam, methionine, lactose, triethanolamine, Tween 80 and phenol in water for injection, adjust the pH to 5.5 with 0.1 mol / L hydrochloric acid solution, and then freeze-dry according to the following steps :
[0037] (1) Pre-freezing stage: freeze the temperature to -18°C within 15 minutes; then heat up to -8°C with a heating time of 60 minutes; freeze the temperature again within 15 minutes to -36°C; then heat up to -26°C, and then heat up The time is 120min; finally, the temperature is frozen to -46℃ again within 15...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com