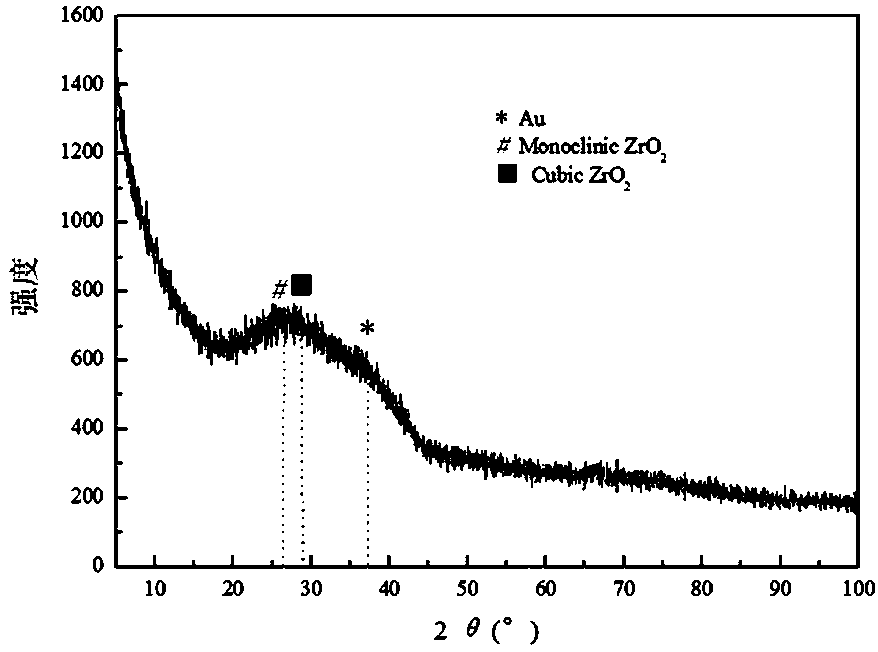
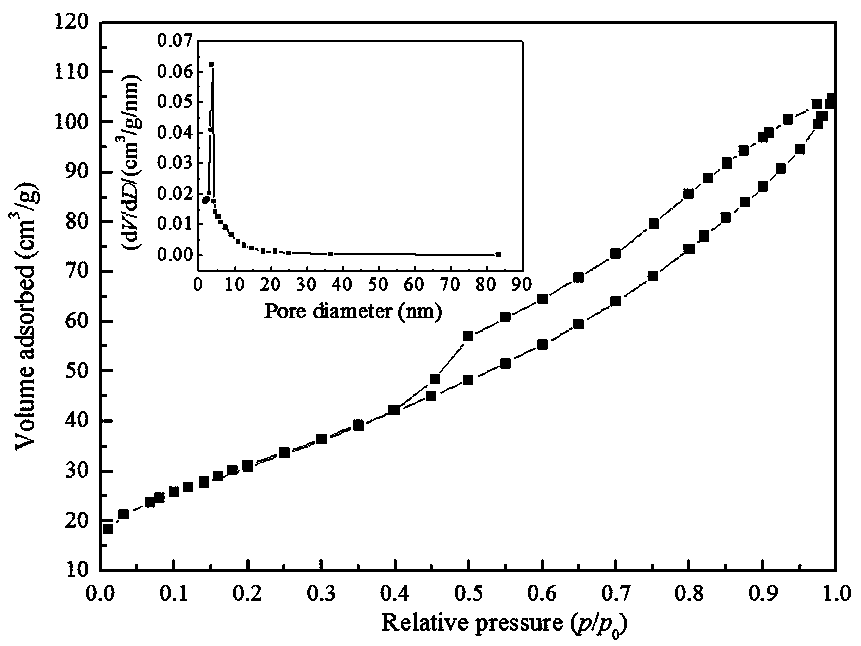
arsenic-removing adsorbent with IB group metal nanoparticles loaded on zinc zirconium composite oxide and preparation method thereof
A metal nanoparticle and composite oxide technology, which is applied in chemical instruments and methods, adsorption water/sewage treatment, water pollutants, etc. The effect of large capacity, less usage and fast adsorption rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] 20 mL of deionized water was used as the co-precipitation bottom solution, and 1.0 M Na 2 CO 3 The pH of the solution was adjusted to 10, and then the temperature was kept constant at 70 °C in a water bath. 0.25 M HAuCl 4 solution, 1.0 M Zn(NO 3 ) 2 solution and 1.0 M ZrOCl 2 ·8H 2 O solution in a certain ratio of substances ( n (Au) / n (Zn) / n (Zr)= 0.05:0.5:1) to prepare mixed metal salt solution. The precipitant uses 1.0 MNa 2 CO 3 solution. Under the condition of magnetic stirring, 46.6 mL of precipitant solution was first added dropwise to the above bottom liquid, and then 24.5 mL of mixed metal salt solution was added dropwise for co-precipitation, and the final pH value was 9~10. After the dropwise addition, continue stirring for 1.0 h to carry out heating and hydrolysis of the precipitate. The hydrolysis temperature is 70° C. and the hydrolysis time is 1 h. After the hydrolysis is completed, take it out and let it stand to cool to room temperature, ...
Embodiment 2
[0040] 20 mL of deionized water was used as the co-precipitation bottom solution, and 1.0 M Na 2 CO 3 The pH of the solution was adjusted to 10, and then the temperature was kept constant at 70 °C in a water bath. 0.25 M HAuCl 4 solution, 1.0 M Zn(NO 3 ) 2 solution and 1.0 M ZrOCl 2 ·8H 2 O solution in a certain ratio of substances ( n (Au) / n (Zn) / n (Zr)= 0.05:0.5:1) to prepare mixed metal salt solution. The precipitant uses 1.0 MNa 2 CO 3 solution. Under the condition of magnetic stirring, 46.6 mL of precipitant solution was first added dropwise to the above bottom liquid, and then 24.5 mL of mixed metal salt solution was added dropwise for co-precipitation, and the final pH value was 9~10. After the dropwise addition, continue stirring for 1.0 h to carry out heating and hydrolysis of the precipitate. The hydrolysis temperature is 70° C. and the hydrolysis time is 1 h. After the hydrolysis is completed, take it out and let it stand to cool to room temperature, ...
Embodiment 3
[0042] 20 mL of deionized water was used as the co-precipitation bottom solution, and 1.0 M Na 2 CO 3 The pH of the solution was adjusted to 10, and then the temperature was kept constant at 70 °C in a water bath. 0.25 M HAuCl 4 solution, 1.0 M Zn(NO 3 ) 2 solution and 1.0 M ZrOCl 2 ·8H 2 The O solution is divided into certain substance ratios ( n (Au) / n (Zn) / n (Zr)=0.13:3:1) to prepare metal salt solution. The precipitant uses 1.0 MNa 2 CO 3 solution. Under the condition of magnetic stirring, 46.6 mL of precipitant solution was first added dropwise to the above bottom liquid, and then 24.5 mL of mixed metal salt solution was added dropwise for co-precipitation, and the final pH value was 9~10. After the dropwise addition, continue stirring for 1.0 h to carry out heating and hydrolysis of the precipitate. The hydrolysis temperature is 70° C. and the hydrolysis time is 1 h. After the hydrolysis is completed, take it out and let it stand to cool to room temperatur...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com