Preparation method of single-phase cobalt nitrate nanomaterial
A technology of cobalt nanometer and niobate, applied in chemical instruments and methods, cobalt compounds, inorganic chemistry, etc., can solve the problems of niobate particle growth, hard agglomeration, niobate grain growth, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
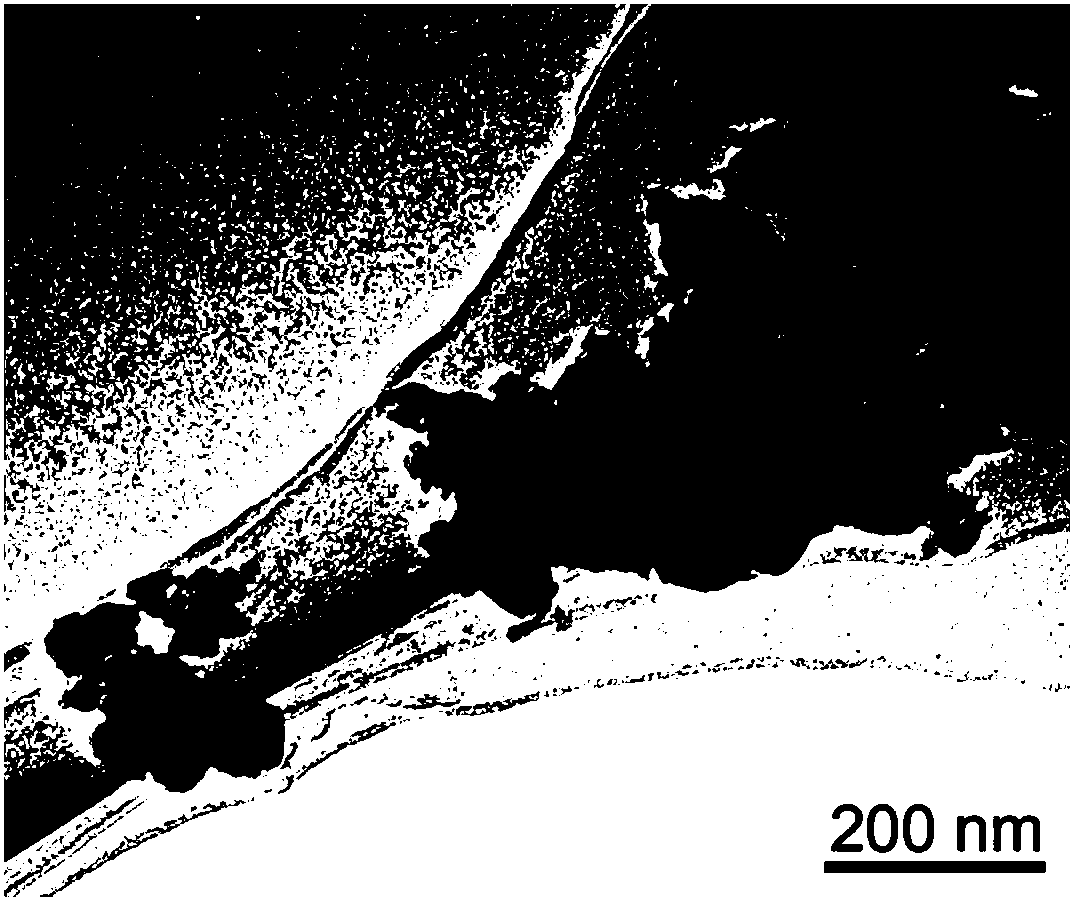
Image
Examples
preparation example Construction
[0021] The invention provides a method for preparing a single-phase cobalt niobate nanomaterial, comprising:
[0022] S1: Dissolving niobium pentachloride and cobaltous chloride in an alcohol solution at a stoichiometric ratio of 2:1 to obtain solution A; dissolving ammonium carbonate in an aqueous solution to obtain solution B. The alcohol solution is one or more of methanol, ethanol, propanol and butanol. The volume ratio of the alcohol solution to the aqueous solution is 2:1˜10:1. The molar concentrations of the niobium pentachloride, cobaltous chloride and ammonium carbonate are all in the range of 1-1000 mmol / L (millimole per liter). Preferably, the niobium pentachloride and cobaltous chloride are dissolved in the alcohol solution by mechanical stirring or ultrasonic stirring, and the ammonium carbonate is dissolved in the aqueous solution by mechanical stirring or ultrasonic stirring.
[0023] S2: Mix solution A and solution B with each other, and stir at room temperat...
Embodiment 1
[0032] Niobium pentachloride and cobaltous chloride are fully dissolved in 300 milliliters of ethanol solutions according to the stoichiometric ratio of 2:1 to obtain solution A, wherein the molar concentration of cobaltous chloride is 40mmol / L; 4 grams of ammonium carbonate are fully Solution B was obtained by dissolving in 100 ml of aqueous solution. Under the premise of sufficient stirring, the solution B was slowly added to the solution A and mixed with each other; after mixing, the mixed solution was stirred at room temperature for 0.5 h to obtain a suspension. The suspension was centrifuged to obtain a powder. The powder was washed 5 times with secondary deionized water and absolute ethanol. Put the cleaned powder into a drying oven, and dry it for one day at a temperature of 50°C. The dried powder was calcined at 700° C. for 8 h to crystallize the powder to obtain a single-phase cobalt niobate nanomaterial.
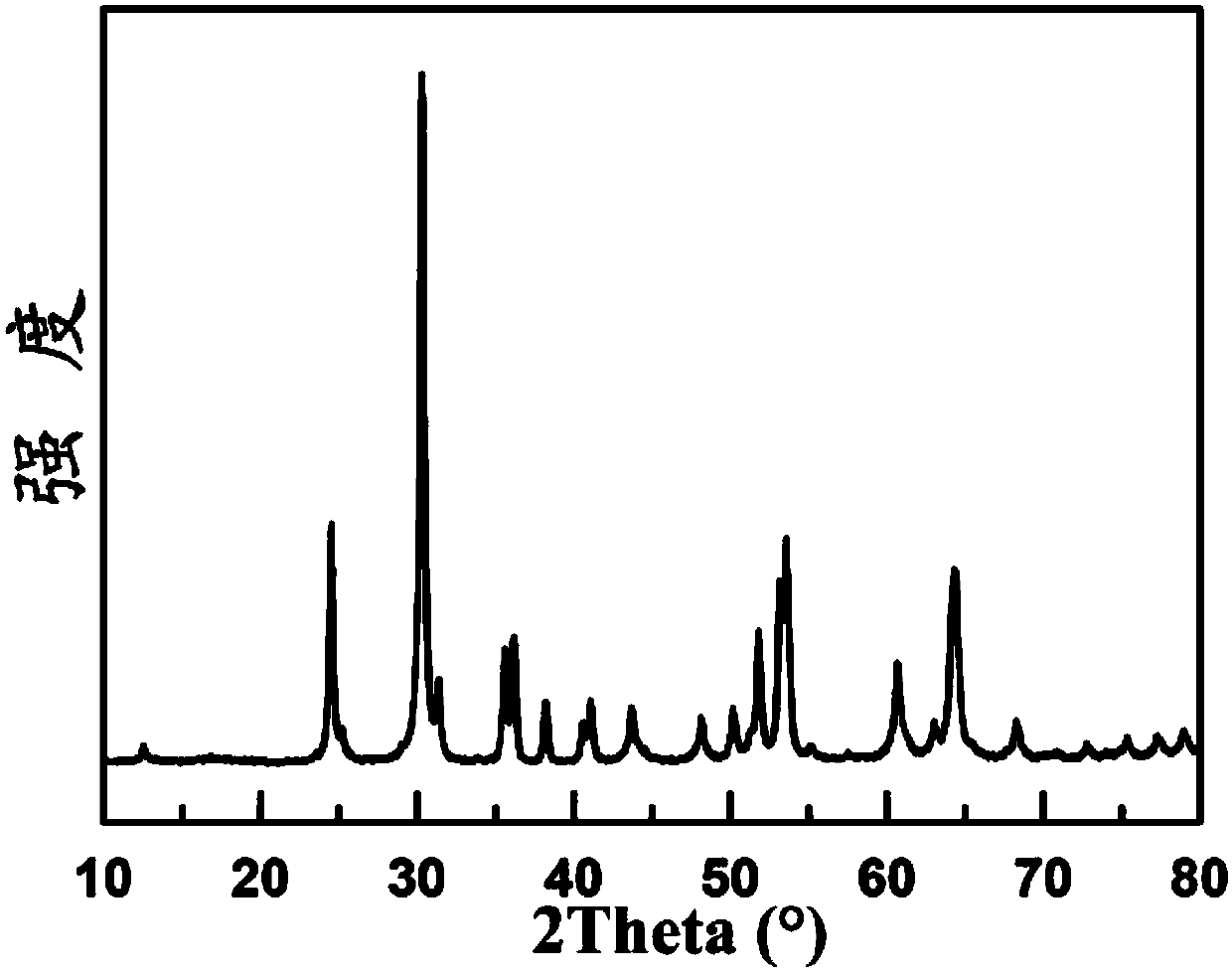
[0033] see figure 1 , figure 1 The X-ray diffraction pat...
Embodiment 2
[0035] Niobium pentachloride and cobaltous chloride are fully dissolved in 500 milliliters of ethanol solutions according to the stoichiometric ratio of 2:1 to obtain solution A, wherein the molar concentration of cobaltous chloride is 30mmol / L; 3.5 grams of ammonium carbonate are fully Solution B was obtained by dissolving in 200 ml of aqueous solution. Under the premise of sufficient stirring, the solution B was slowly added to the solution A and mixed with each other; after mixing, the mixture was stirred at room temperature for 1 h to obtain a suspension. The suspension was centrifuged to obtain a powder. The powder was washed 5 times with secondary deionized water and absolute ethanol. Put the cleaned powder into a drying oven and dry it at 60°C for one day. The dried powder was calcined at 700° C. for 10 h to crystallize the powder to obtain a single-phase cobalt niobate nanomaterial.
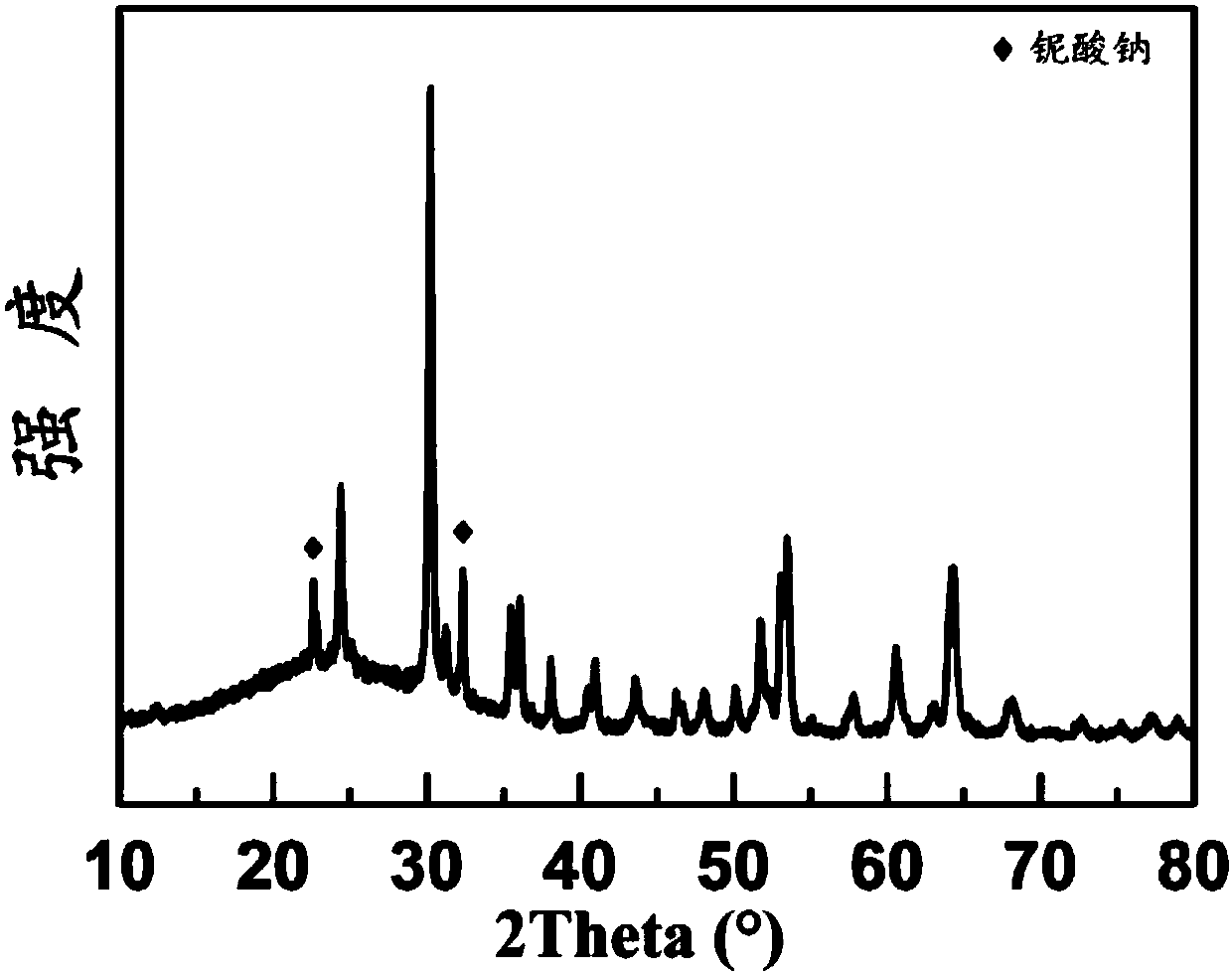
[0036] It can be seen from the experimental test that the single-phase cobalt niob...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com