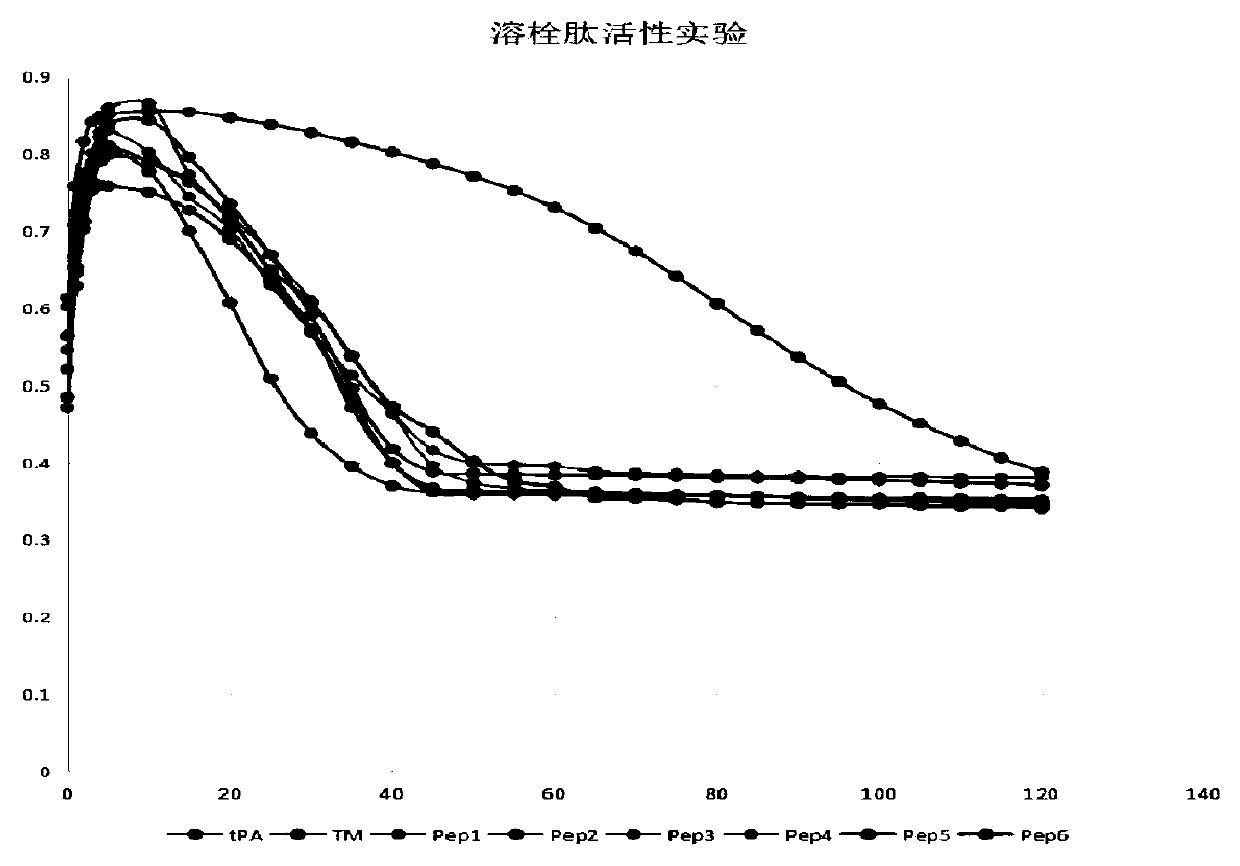
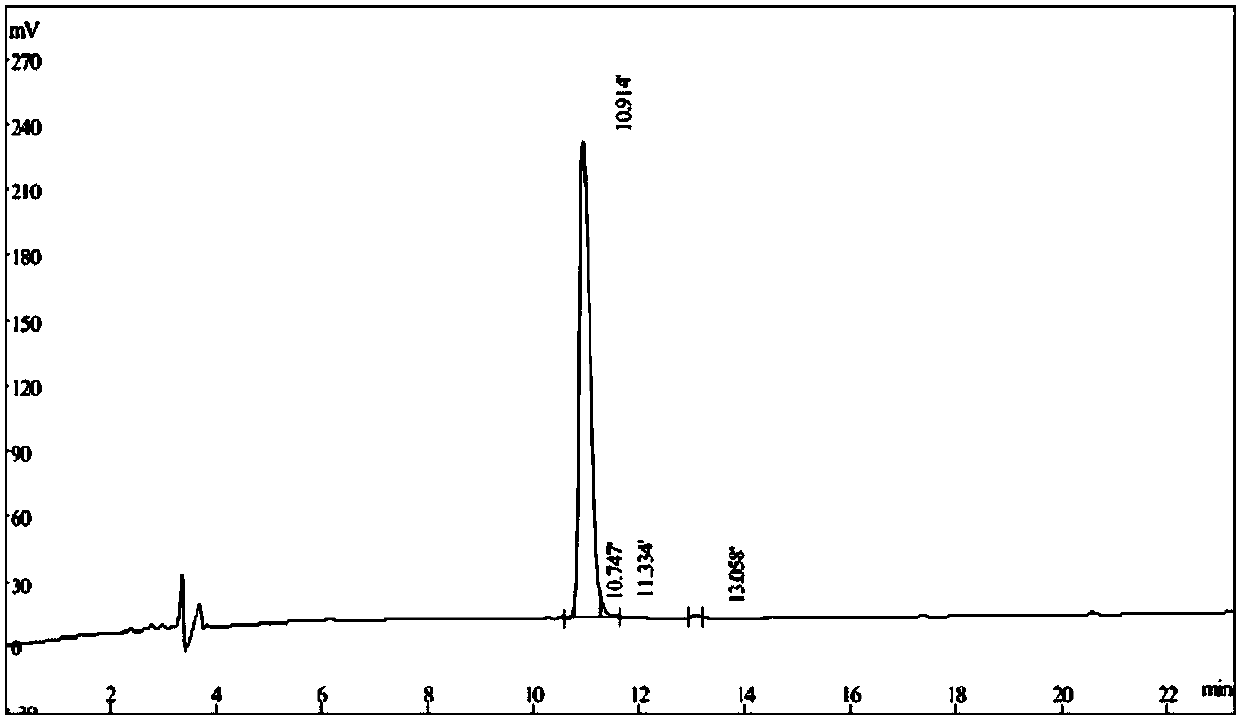
Polypeptide with thrombolytic activity
A thrombus and thrombolytic technology, applied in the field of peptides with thrombolytic activity, can solve the problems of affecting the effect of thrombolytic therapy, narrow safe dose range, and fatal bleeding complications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 Screening of Thrombolytic Active Polypeptides
[0033] 1. Design screening database
[0034] 1) Establish a full library of random primary hexapeptide databases: X-X-X-X-X-X. Wherein X is Fmoc protected D-Gly, D-Ala, D-Val, D-Leu, D-Ile, D-Phe, D-Pro, D-Tyr, D-Ser, D-Thr, D-Trp, D-Met, D-Glu, D-Gln, D-Asp, D-Asn, D-Lys, D-Arg, D-His equimolar mixture.
[0035] 2) Establish a secondary hexapeptide random database: Ile-Thr-X-X-X-X and Glu-Asp-X-X-X-X. Wherein X is Fmoc protected D-Gly, D-Ala, D-Val, D-Leu, D-Ile, D-Phe, D-Pro, D-Tyr, D-Ser, D-Thr, D-Trp, D-Met, D-Glu, D-Gln, D-Asp, D-Asn, D-Lys, D-Arg, D-His equimolar mixture.
[0036] 3) Establish a tertiary hexapeptide random database: Ile-Thr-Met-Ala-X-X-X-X and Glu-Asp-Ser-Arg-X-X-X-X. Wherein X is Fmoc protected D-Gly, D-Ala, D-Val, D-Leu, D-Ile, D-Phe, D-Pro, D-Tyr, D-Ser, D-Thr, D-Trp, D-Met, D-Glu, D-Gln, D-Asp, D-Asn, D-Lys, D-Arg, D-His equimolar mixture.
[0037] 4) The polypeptide combination ...
Embodiment 2
[0047] Synthesis and purification of embodiment 2 polypeptide
[0048] Synthesis Polypeptides were synthesized from C-terminus to N-terminus by solid-phase synthesis. Synthesis using a chemical synthesizer (AMS586Multiple Peptide Synthesiser, ABIMED, Germany), using Fmoc-protected amino acids as raw materials, using Fmoc-Rink linker resin resin as an attachment matrix, using HOBT as a condensation agent, and DIC as an activator to synthesize peptides layer by layer .
[0049] During the synthesis process, 2% acetic anhydride in DMF solution was used as the side chain blocking reagent; 20% piperidine was used as the Fmoc removal reagent, and after the synthesis was completed, TFA cleavage and removal of side chain groups were performed. The synthesized crude product was collected by centrifugation, and was analyzed by R-HPLC (Waters 741) and C 18 -column(Waters Delta-pak TM , 40*100mm, 15um, 100 angstroms) was purified to obtain a polypeptide with a purity greater than 98%, ...
Embodiment 3
[0058] Embodiment 3 The animal experiment of polypeptide thrombolytic activity
[0059] 1 Thrombolysis experiment in vivo
[0060] Experimental method: 200 Wistar rats were selected and randomly divided into 20 groups, 10 rats in each group, which were blank control group, positive control group, low-, medium-, and high-dose groups (test polypeptide samples were respectively 0.25, 0.5, 1.0 mg / kg), the positive drug group was given urokinase 3000U / kg, and the control group was given the same volume of normal saline. After 45 minutes of intravenous administration, each group was anesthetized by intraperitoneal injection of 3% pentobarbital sodium (1ml / kg), and electrically stimulated to injure the carotid artery of rats to form thrombus, and the thrombus formation time was observed with a thrombus analyzer. The experimental results are shown in Table 3.
[0061] Table 3 Thrombolysis experiment results in vivo
[0062]
[0063]
[0064] ※ P<0.05 There is a statistical d...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap