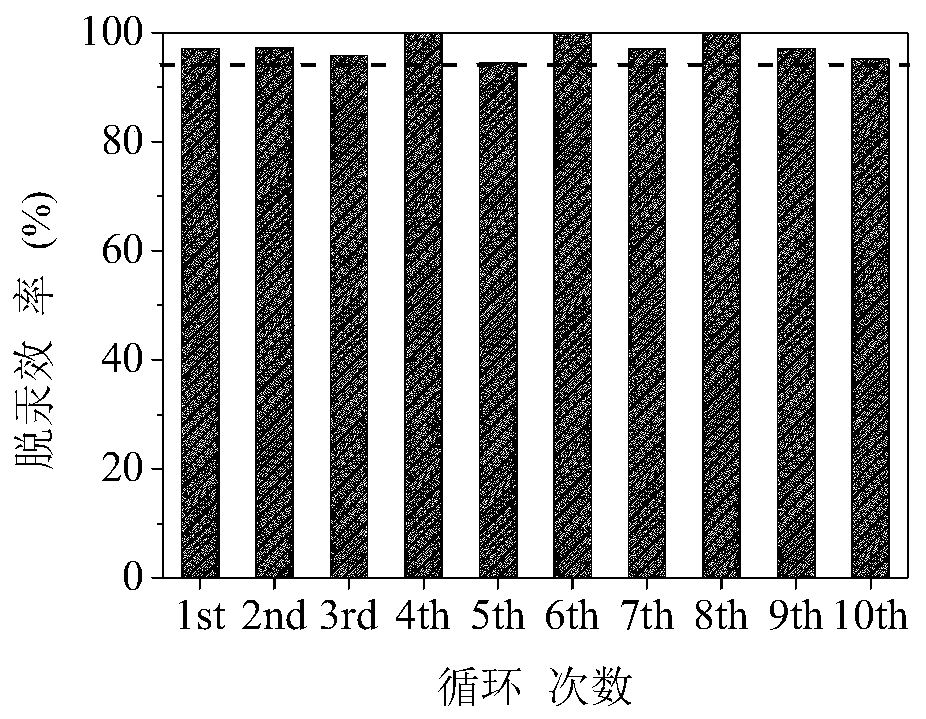
A kind of regenerable recycling mercury adsorbent and its preparation and regeneration method
A technology of adsorbent and elemental mercury, which is applied in the field of mercury adsorbent and its preparation and regeneration, can solve the problems that mercury cannot be centrally controlled, unfavorable for fly ash recycling, and affects the carbon content of fly ash, etc., to achieve large-scale production , Reduce the cost of mercury removal, the effect of short production cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
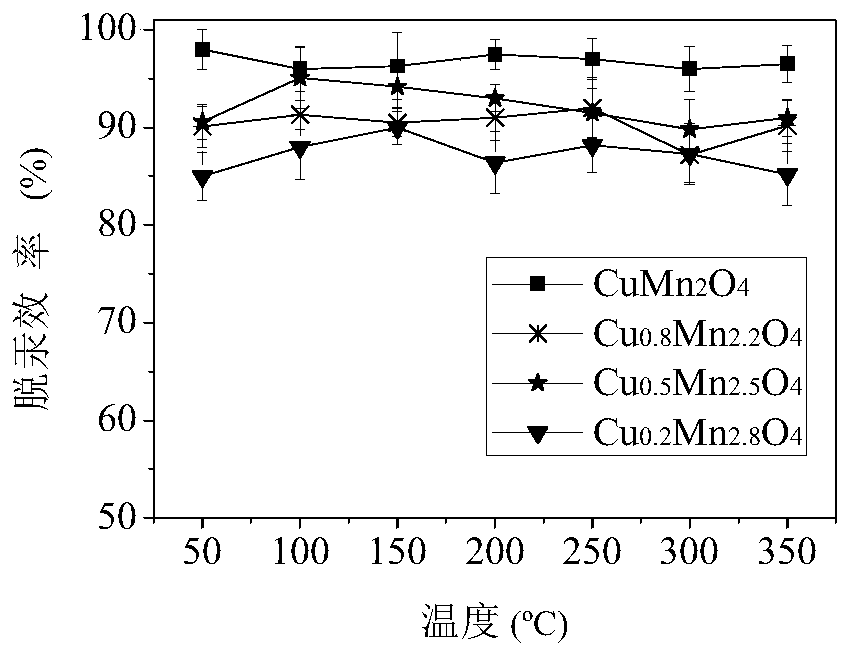
[0033] A renewable and recyclable mercury sorbent with the chemical formula CuMn 2 o 4 . Its preparation method comprises the following steps:
[0034] (1) Weigh a certain amount of copper nitrate and citric acid and dissolve them in deionized water.
[0035] (2) Then, under the condition of stirring, in the mixed solution that step (1) obtains, add a certain amount of manganese nitrate so that the molar ratio of Cu / Mn is 1:2, and the molar ratio of metal cation / citric acid is 1, React for 1 h at 50°C.
[0036] (3) Stir and evaporate in a water bath at 80° C. until a colloid is formed, and dry the colloid in a drying oven at 100° C. for 10 hours. Then, it was calcined in a muffle furnace at 400°C for 3 hours, so as to ensure that the citric acid in the colloid was completely burned and released in the form of gas.
[0037] (4) Grinding and sieving the calcined sample to obtain CuMn with a particle size less than 150 μm 2 o 4 Spinel mercury sorbent.
Embodiment 2
[0039] A renewable and recyclable mercury sorbent with the chemical formula Cu 0.8 mn 2.2 o 4 . Its preparation method comprises the following steps:
[0040] (1) Weigh a certain amount of copper nitrate and citric acid and dissolve them in deionized water.
[0041] (2) Then, under the condition of stirring, add a certain amount of manganese nitrate to the mixed solution that step (1) obtains so that the molar ratio of Cu / Mn is 0.8:2.2, and the molar ratio of metal cation / citric acid is 1.25, React at 60°C for 2h.
[0042] (3) Stir and evaporate in a water bath at 90° C. until a colloid is formed, and dry the colloid in a drying oven at 110° C. for 11 hours. Then, it was calcined in a muffle furnace at 425°C for 4 hours, so as to ensure that the citric acid in the colloid was completely burned and released in the form of gas.
[0043] (4) Grind and sieve the calcined sample to obtain Cu with a particle size of less than 150 μm 0.8 mn 2.2o 4 Spinel mercury sorbent.
Embodiment 3
[0045] A renewable and recyclable mercury sorbent with the chemical formula Cu 0.5 mn 2.5 o 4 . Its preparation method comprises the following steps:
[0046] (1) Weigh a certain amount of copper nitrate and citric acid and dissolve them in deionized water.
[0047] (2) Then, under the condition of stirring, add a certain amount of manganese nitrate to the mixed solution that step (1) obtains so that the molar ratio of Cu / Mn is 0.5:2.5, and the molar ratio of metal cation / citric acid is 1.5, React at 70°C for 3h.
[0048] (3) Stir and evaporate in a water bath at 95° C. until a colloid is formed, and dry the colloid in a drying oven at 115° C. for 11.5 hours. Then, it was calcined in a muffle furnace at 450°C for 4.5 hours, so as to ensure that the citric acid in the colloid was completely burned and released in the form of gas.
[0049] (4) Grind and sieve the calcined sample to obtain Cu with a particle size of less than 150 μm 0.5 mn 2.5 o 4 Spinel mercury sorbent....
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com