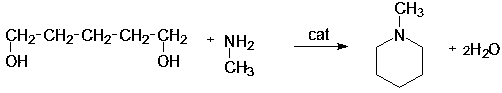Preparation method for amination synthesis of N-methylpiperidine from 1,5-pentanediol
A technology of methylpiperidine and pentanediolamine, which is applied in the fields of chemical instruments and methods, organic chemistry, molecular sieve catalysts, etc., can solve the problems of catalyst recycling, increased industrial production costs, and high requirements for reaction equipment, and achieve high The value of industrialized production, the effect of reducing hidden dangers in production safety, reducing corrosion and environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] A method for preparing N-methylpiperidine by amination of 1,5-pentanediol. The molar ratio of raw materials 1,5-pentanediol and monomethylamine is 1:1, and the liquid hourly space velocity of the raw materials is 0.5h -1 , the water content of 1,5-pentanediol in the raw material is 0.1wt%, and the above-mentioned raw material is continuously fed into the tubular reactor filled with the upper and lower layers of catalyst through the metering pump, and the reaction temperature in the tubular reactor is 220 ℃, the reaction pressure is 0.5Mpa, carry out amination reaction to obtain the crude product, and then obtain N-methylpiperidine through vacuum distillation and purification, wherein the vacuum degree in the column of vacuum distillation is -0.09MPa, and the obtained N-methylpiperidine The purity of the piperidine was 98.5wt%, and the water content was 0.06wt%.
[0027] The main reaction formula for the synthesis of N-methylpiperidine by the amination reaction of the ab...
Embodiment 2
[0032] This example is basically the same as Example 1, except that the molar ratio of the raw materials 1,5-pentanediol and monomethylamine is 1:1.1, and the liquid hourly space velocity of the raw materials is 0.75h -1 , the water content of 1,5-pentanediol in the raw material is 0.1wt%. The above-mentioned raw material is continuously fed into the tubular reactor filled with the upper and lower layers of catalyst through the metering pump. The reaction temperature in the tubular reactor is 230 ℃, the reaction pressure is 1.0Mpa, the amination reaction is carried out to obtain the crude product, and then the N-methylpiperidine is obtained through vacuum distillation and purification, the content of the obtained N-methylpiperidine is 99.0wt%, and the water content is 0.05wt %.
[0033] The active component of the supported catalyst is a solid superacid, and the active component in the solid superacid is SO 4 2- , the carrier of the supported catalyst is alumina, titania, ir...
Embodiment 3
[0035] This example is basically the same as Example 1, except that the molar ratio of the raw materials 1,5-pentanediol and monomethylamine is 1:2.0, and the liquid hourly space velocity of the raw materials is 2h -1 , the water content of 1,5-pentanediol in the raw material is 0.1wt%, and the above-mentioned raw material is continuously fed into the tubular reactor filled with the upper and lower layers of catalyst through the metering pump, and the reaction temperature in the tubular reactor is 260 ℃, the reaction pressure is 2.0Mpa, carry out amination reaction to obtain the crude product, and then obtain N-methylpiperidine through vacuum distillation and purification, the content of the obtained N-methylpiperidine is 98.5wt%, and the water content is 0.05wt %.
[0036] The active component of the supported catalyst is a solid superacid, and the active component in the solid superacid is PO 4 3- , the carrier of the supported catalyst is alumina, titania, vanadium oxide ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| flash point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com