Babesia microti thioredoxin molecule and its gene and application
A technology of babesia thioredoxin and thioredoxin, applied in the field of bioengineering, can solve problems such as unstable effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
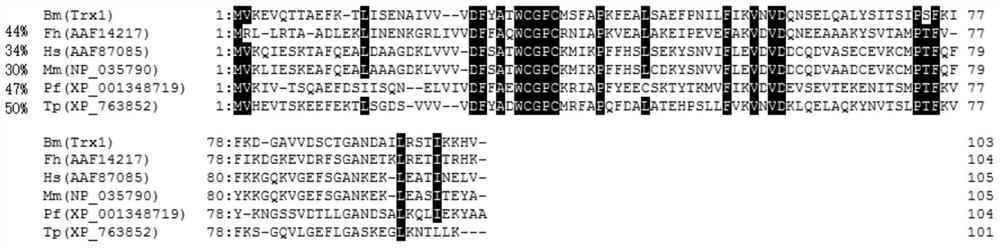
[0027] Example 1 Gene Cloning and Sequence Analysis of Babesia microti thioredoxin molecule (BmTrx1)
[0028] 1. Materials and methods
[0029] 1.1. Parasites and experimental animals
[0030] Babesia microti was purchased from ATCCR PRA-99TM under the American Standard Culture Collection (ATCC), and was subcultured and preserved in Kunming mice in our laboratory.
[0031] 1.2. Bacteria and plasmids
[0032] Escherichia coli DH5α (Invitrogen) and Escherichia coli BL21(DE3) (Novagen) were used for plasmid construction and protein expression. The sequencing vector was pGEM-T Easy (Promega), and the expression vector was pET30a vector (Novagen).
[0033] 1.3. Molecular cloning of Babesia microti thioredoxin (BmTrx1)
[0034] The mouse erythrocytes infected with Babesia microti were collected, crushed, and the worms were collected by centrifugation. The parasite RNA was extracted with TRIzolreagent (Invitrogen), and then the genomic DNA of the parasite was removed with DNase ...
Embodiment 2
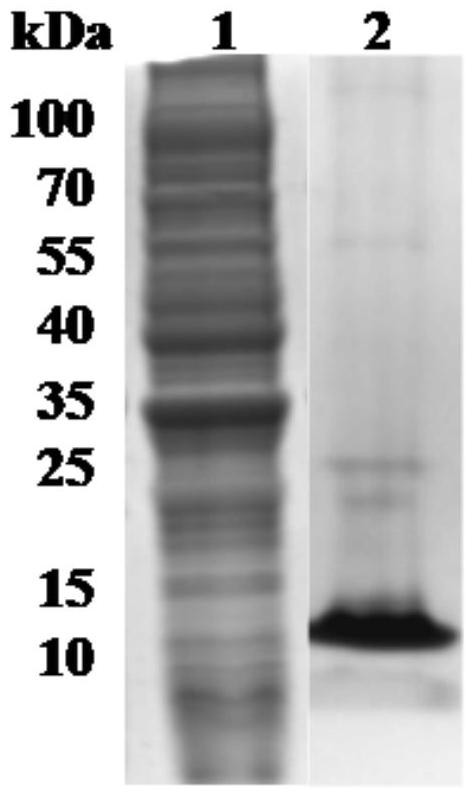
[0037] Example 2 Recombinant expression and purification of Babesia microti thioredoxin molecule (BmTrx1)
[0038] In order to reduce the influence of exogenous amino acids on the protease activity of BmTrx1, the target gene BmTrx1 was cloned into the expression vector pET30a, and only the His tag was introduced at the C-terminus, and then expressed in E.coli BL21.
[0039] With pET-Trx1-F:5'-AAGGAGATATA CATATGGTGAAAGAAGTACAAACTACTGCTGA-3' (SEQ ID NO. 8) and pET-Trx1-R: 5'-GGTGGTGGTG CTCGAG GACGTGCTTCTTGATAGTGCTCC-3'(SEQ ID NO.9) is the expression primer amplified to obtain the open reading frame sequence of BmTrx1, which is connected to the enzyme-digested (Nde I and Xho I) by In-Fusion HD Cloning Kit (Takara) in one step Expression vector pET-30a (Novagen), and transfected with DH5α. After the positive clones were obtained by sequencing, the positive plasmids were extracted and transfected into E.coli BL21(DE3) for protein expression. At 37°C, 1mMIPTG was induced for 8 ...
Embodiment 3
[0042] Example 3 Preparation of recombinant protein rBmTrx1 / His antiserum and detection by Western Blotting
[0043] After the recombinant protein rBmTrx1 / His was thoroughly mixed with an equal volume of Freund's complete adjuvant, about 100ug of protein per mouse was injected intraperitoneally. On the 14th day and 28th day after the initial immunization, booster immunization was carried out twice with fully mixed recombinant protein and incomplete adjuvant. The anti-rBmTrx1 / His serum of mice was collected after the antibody titer was determined by ELISA.
[0044] RBCs from mice infected with Babesia microti (2 to 10 days) and uninfected (0 days) were electrophoresed on a 15% SDS-PAGE gel, then transferred to Immobilon-P SQ membrane (Millipore). After blocking with 5% skimmed milk overnight at 4°C, the membrane was reacted with the collected anti-rBmTrx1 / His serum (diluted 1:200) at room temperature for 1 hour, and then washed 3 times with PBST, 10 minutes each time. After ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com