Tedizolid phosphate composition tablet
A technology of tedizolid phosphate and composition, applied in the field of tedizolid phosphate composition tablet, can solve the problems of inability to meet transportation and storage, unstable product quality, inaccurate dosage form, etc. Application, low cost, low loss effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
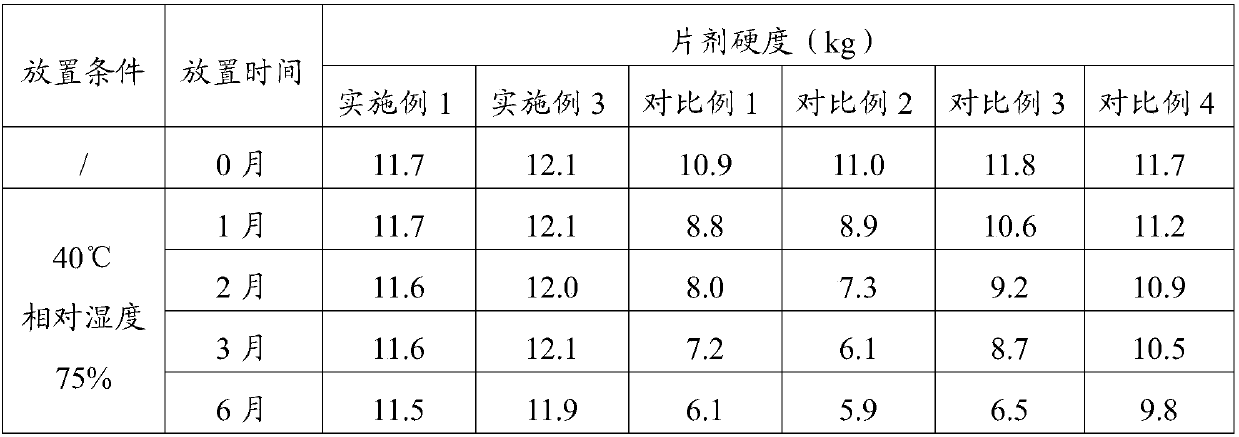
[0046] Experimental Example 1 Investigation of tablet hardness and stability
[0047] Take the tablet samples prepared in Example 1, Example 3, Comparative Example 1, Comparative Example 2, Comparative Example 3, and Comparative Example 4, under the conditions of packaging (aluminum-plastic packaging, placed in a carton), at 40 ° C, It was placed under a relative humidity of 75% for 6 months, and samples were taken at 0, 1, 2, 3, and 6 months to measure tablet hardness. The results are shown in Table 1.
[0048] Table 1 Tablet hardness test results
[0049]
[0050] The accelerated test is investigated for 6 months, and the hardness measurement results show that the hardness of the tablet of the present invention remains unchanged under long-term storage conditions, which significantly improves the stability of the formulation.
experiment example 2
[0051] Experimental example 2. Dissolution detection
[0052] Dissolution determination method: The test and reference substances were tested for dissolution according to the second appendix XD of the 2015 edition of the Pharmacopoeia of the People's Republic of China. Take 900ml of 0.05M phosphate buffer, pH 6.80±0.05 as the dissolution medium, the temperature is 37°C, the rotation speed is 50 rpm, and the amount of drug in each sample to be tested is 30mg. Dissolution was measured by UV spectrophotometry at 45min. The detection wavelength was 274nm, and the linear relationship was good within 5~25mg·L-1. The recovery and precision experiments all met the methodological requirements. The test results are shown in Table 2.
[0053] Table 2 Dissolution test results
[0054]
experiment example 3
[0055] Experimental Example 3: Efficacy and Safety Evaluation of the Treatment of MRSA Pneumonia
[0056] Refer to the method of patent CN 105085570 A for detection, and the detection results are shown in Table 3-5.
[0057] Table 3 Comparison of clinical efficacy of patients (cases)
[0058] group
[0059] Table 4 Comparison of bacteriological efficacy of patients (cases)
[0060] group
[0061] Table 5 Comparison of adverse reactions in two groups of patients (cases)
[0062] group
[0063] The comparison difference between the embodiment and the comparative example has statistical significance (P<0.05), thus it can be seen that the curative effect and adverse reaction of the tedizolid phosphate composition of the present invention are superior to the prior art, and have significant antibacterial activity to MRSA. improve.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com