A hard capsule preparation
A technology of hard capsules and preparations, which is applied in capsule delivery, medical preparations containing non-active ingredients, and medical preparations containing active ingredients. Excellent stability and uniform drug content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Preparation of mannitol particles:
[0020] After taking 4 kg of mannitol through a 200-mesh sieve, it is placed in a high-shear wet granulation pot with a stirring speed of 500 rpm / min, a cutting speed of 3000 rpm, and 800 g of water for granulation at a rate of 400 g / min. After the addition, continue to shear for 1 min, take out the wet mannitol granules and dry them at 65°C until the moisture content is less than 0.3%. The granules are sized through a 24-mesh sieve as the low-water active auxiliary material granules.
Embodiment 2
[0022] Preparation of mannitol particles:
[0023] Take 200SD direct pressure grade mannitol, dry it in a constant temperature drying oven at 65°C until the moisture content is less than 0.3%, and take it out through a 40-mesh sieve as the low-water activity auxiliary material particles.
Embodiment 3
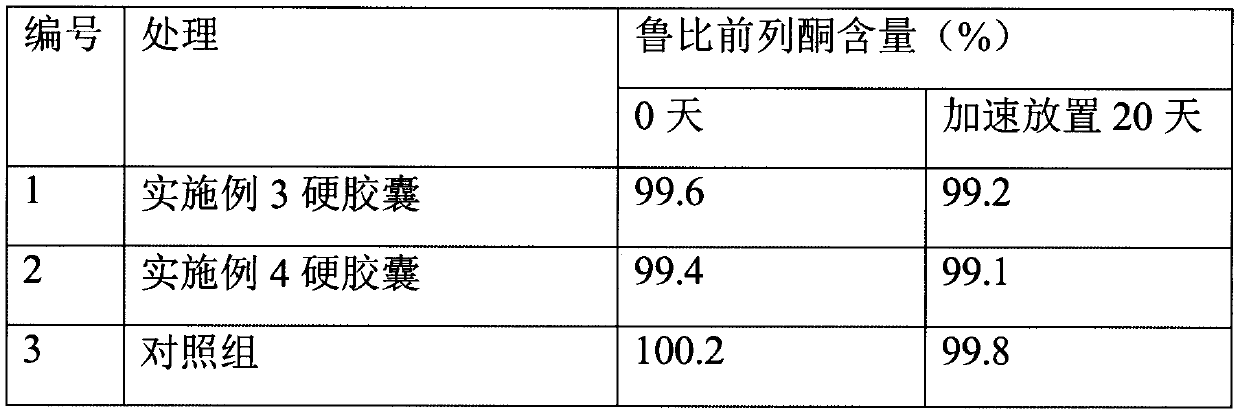
[0025] Preparation of Lubiprostone Hard Capsules
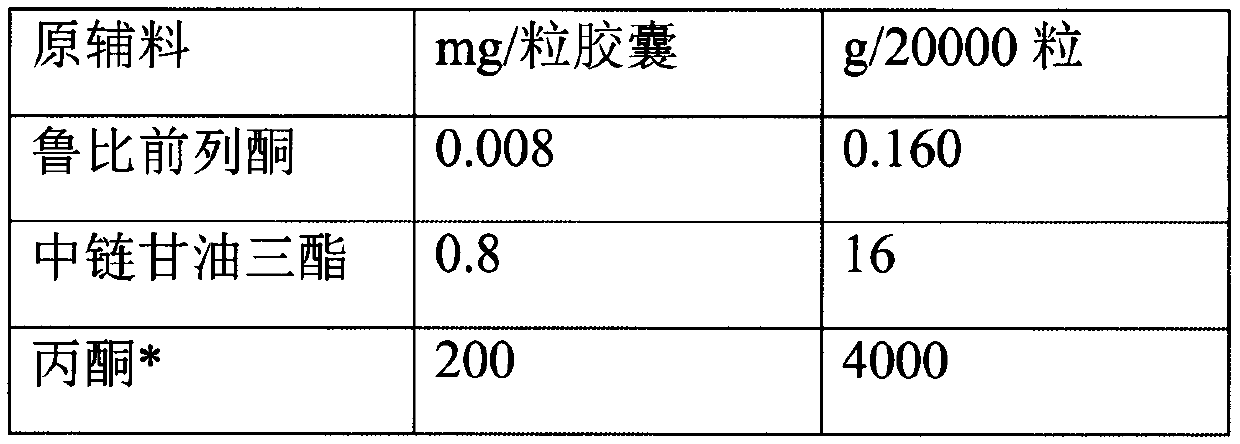
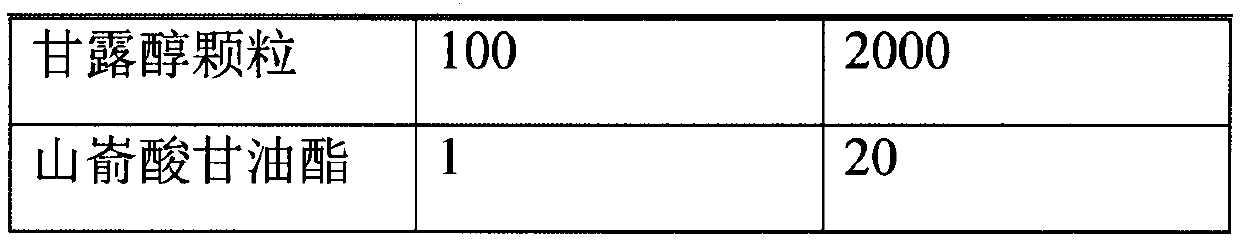
[0026] Prepare according to the following prescription:
[0027]
[0028]
[0029] *Volatilized and removed during the preparation process.
[0030] Put the prescription amount of lubiprostone and medium-chain triglycerides in the prescription amount of acetone, stir and dissolve at 20 rpm, add mannitol particles, continue stirring, open the preparation tank and heat it at 40℃, turn on the vacuum device, and acetone The volatilization pipeline on the top of the preparation tank enters the acetone collection tank. When the collection continues until there is no fraction, the vacuum is continued for 1 hour, the particles are taken out, the organic solvent is detected, the prescription amount of glyceryl behenate is added, and the three-dimensional mixer is mixed for 3 minutes at 25 rpm. Control the drug content in the granules and then fill them into No. 3 hard capsules
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com