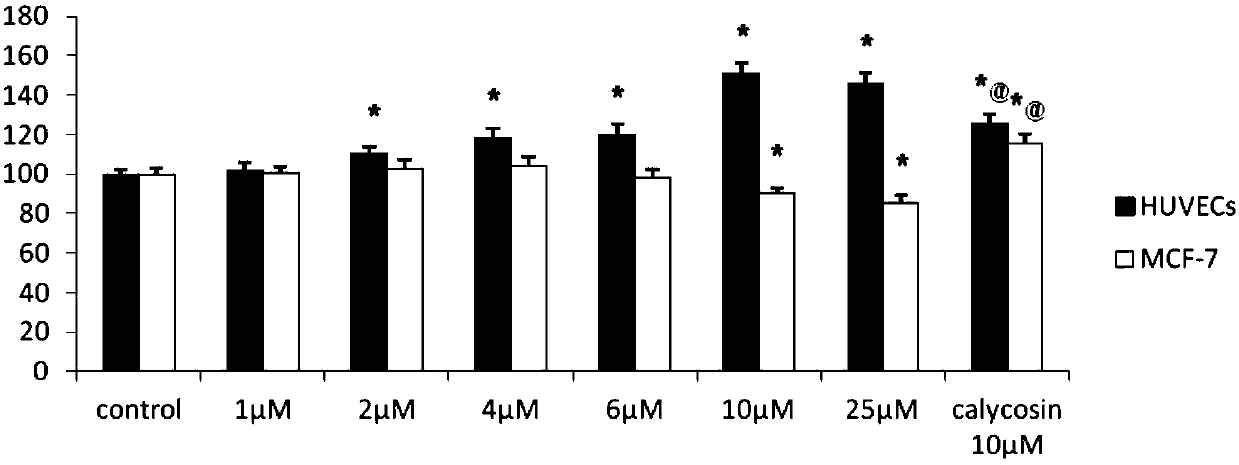
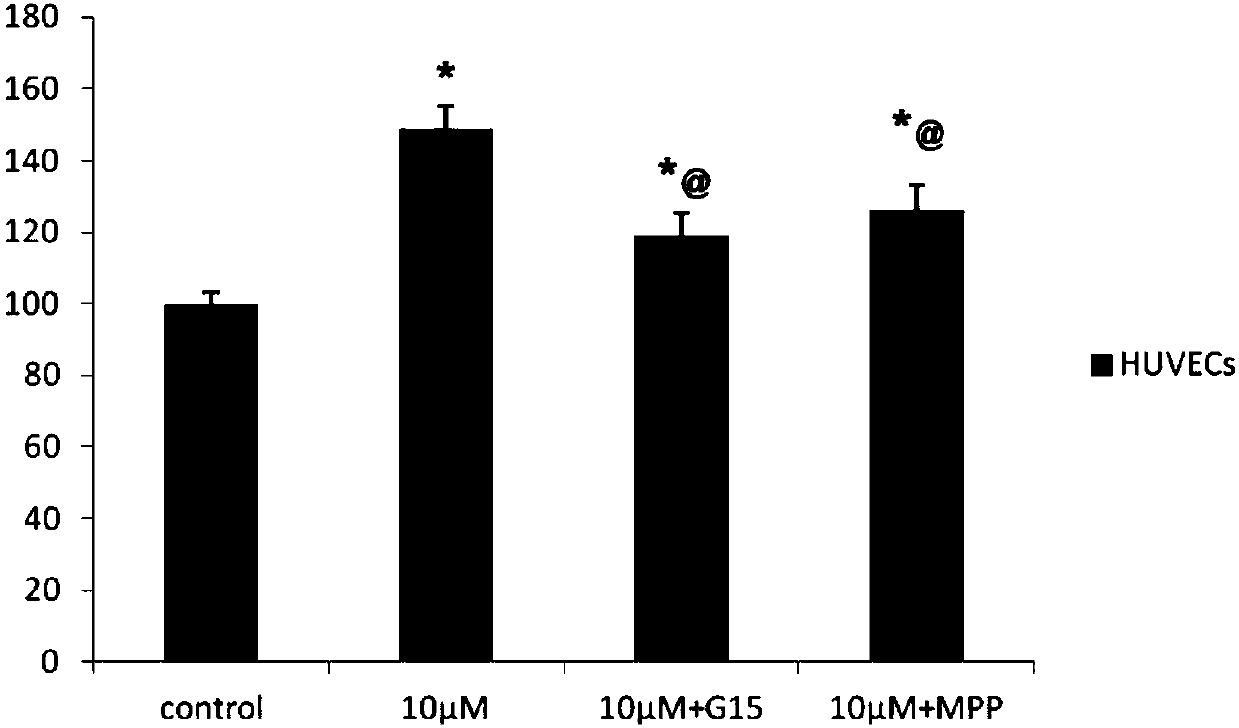
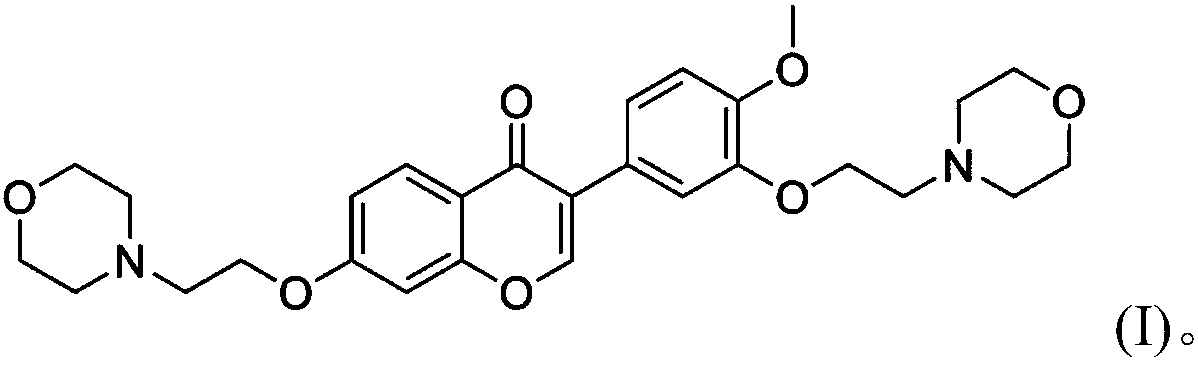
Calycosin derivative and synthesis method thereof
A synthesis method and product technology, applied in the field of medicine, can solve the problems of unclear target and large effective concentration, achieve good vascular protection effect, and promote the effect of human umbilical vein endothelial cell proliferation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] 1) Take 7-hydroxy-3-(3-hydroxy-4-methoxy)-4H-benzopyran-4-one (300mg) and 1,2-dibromoethane in a molar ratio of 1:8 Dissolve in 13mL of acetone, then adjust the pH of the system to 10 with triethylamine, and react for 8h at room temperature. After the reaction is over, spin dry, add 200ml of ethyl acetate to the residue, and wash with 300mL*3 of water three times. Afterwards, it was dried with anhydrous sodium sulfate and purified on a silica gel column (eluent: ethyl acetate / petroleum ether=1:4, volume ratio) to obtain intermediate product 3 (yield 60%);
[0032] 2) Dissolve intermediate product 3 (100mg) and morpholine in 10mL acetone at a molar ratio of 1:1, adjust the pH of the system to 10 with triethylamine, and react at room temperature for 6 hours. After the reaction is complete, spin-dry it. 150ml ethyl acetate was added to the obtained residue, washed three times with 200ml*3 water, dried with anhydrous sodium sulfate, purified on a silica gel column (eluent: eth...
Embodiment 2
[0039] Repeat Example 1, the difference is that the organic solvent in steps 1) and 2) is changed to DMF. A yellow solid product was obtained (yield 55%).
[0040] The yellow solid product obtained in this example was characterized by nuclear magnetic resonance and determined to be the target compound of the application.
Embodiment 3
[0042] Repeat Example 1, except that the organic solvent in steps 1) and 2) was changed to chloroform. A yellow solid product was obtained (yield 20%).
[0043] The yellow solid product obtained in this example was characterized by nuclear magnetic resonance and determined to be the target compound of the application.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com