Chlorambucil precursor compound with photo-responsive degradation effects and its preparation and application
A kind of precursor compound, the technology of leuconin, which is applied in the field of leuconin precursor compounds, can solve the problems of toxic and side effects, incomplete and burst release of leuconin preparations, unable to release at a fixed time and at a fixed point, etc., and achieves the drug load Large, small size, reducing the effect of toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1: the preparation of precursor compound molecule
[0031] Dissolve 180mg (0.6mmol) of Cyclonine in 10mL of anhydrous CH 2 Cl 2 After cooling in an ice bath, add 148mg (0.72mmol) of N,N'-dicyclohexylcarbodiimide, 13.8mg (0.072mmol) of 4-dimethylaminopyridine and 236mg (0.6mmol) of hexadecyl For nitrobenzyl derivatives, react at 0-5°C for 1 hour, then naturally rise to room temperature and keep reacting for 24 hours. After the reaction was finished, the white by-product (DCU) was filtered off, the filtrate was concentrated under reduced pressure, and the white solid product was obtained by silica gel column chromatography. The eluent was ethyl acetate:petroleum ether, and the yield was 75%.
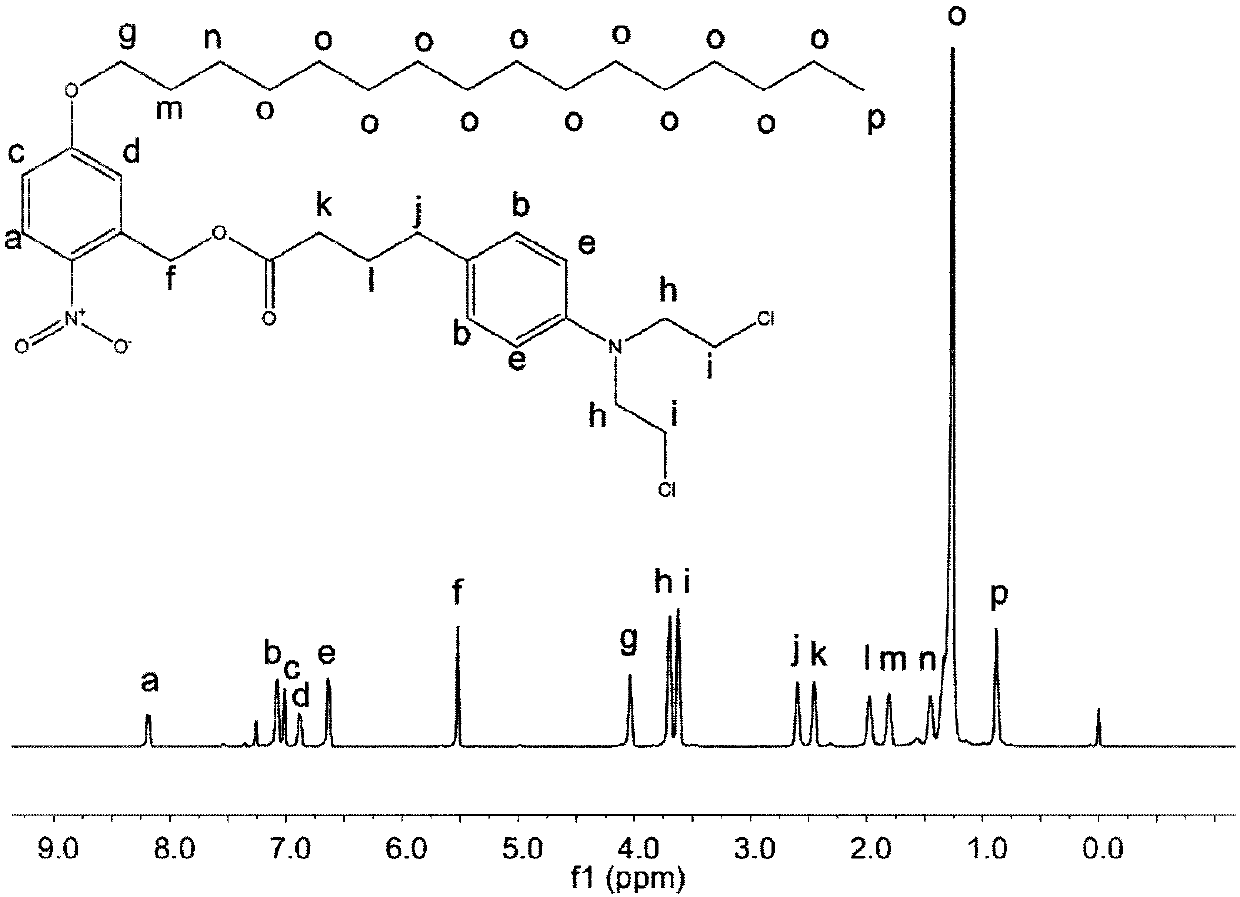
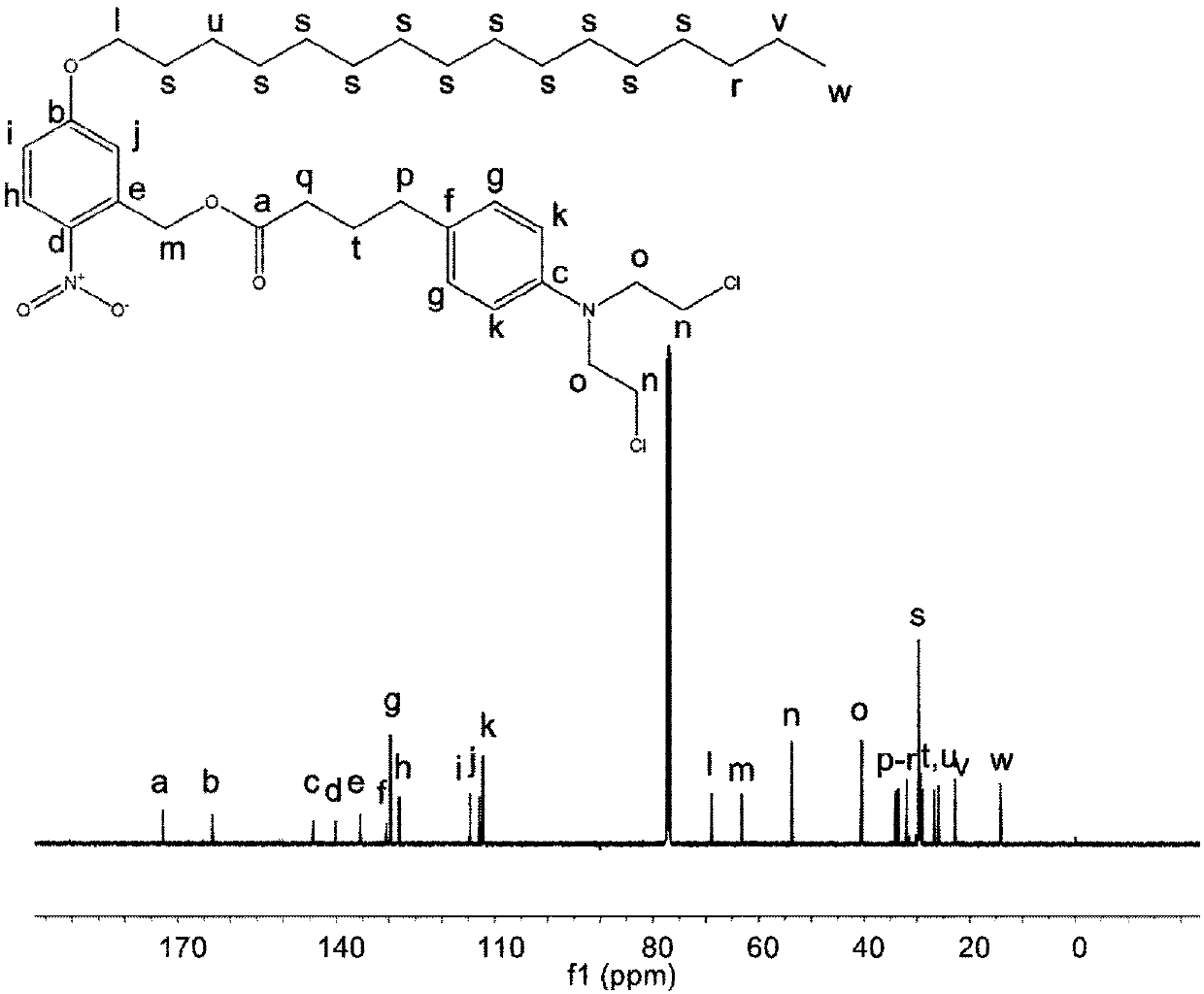
[0032] The molecular structure of the precursor compound was characterized by H NMR and C NMR results. The results are as follows: figure 1 and figure 2 shown.
Embodiment 2
[0033] Example 2: Photodegradation Assay of Precursor Compound Molecules
[0034] Prepare 0.1mg / mL acetonitrile solution of precursor compound molecules, and irradiate with 365nm ultraviolet light (1mW / cm 2 ) irradiated for different times, using high-performance liquid chromatography to measure the behavior of precursor compound molecules transformed into Lianke Ning drug molecules by light, and the degradation efficiency was determined by comparing the integral areas of the two. The results are as follows image 3 shown. Experiments have shown that the precursor compound molecules are almost completely degraded after only 10 minutes of irradiation.
Embodiment 3
[0035] Embodiment 3: the preparation of nano drug
[0036] Precursor compound molecules (20 mg) and MSNs (18 mg) were dissolved in 3 mL of absolute ethanol, shaken at room temperature for 24 hours, centrifuged at 10,000 rpm for 10 minutes to collect nanoparticles, and washed twice with water to obtain nanomedicines. The morphology of the nanomedicine was characterized by transmission electron microscopy, and the results were as follows: Figure 4 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com