Preparation method of ruxolitinib intermediate (3R)-3-(4-Br-1H-pyrazole-1-yl)-cyclopentyl propanenitrile
A technology of cyclopentylpropionitrile and cyclopentylpropionitrile, which is applied in the field of preparation of ruxolitinib intermediate-3--cyclopentylpropionitrile, can solve API isomers with many impurities and high cost , not easy to obtain and other problems, to achieve the effect of high stereoselectivity, mild reaction conditions and good stereoselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
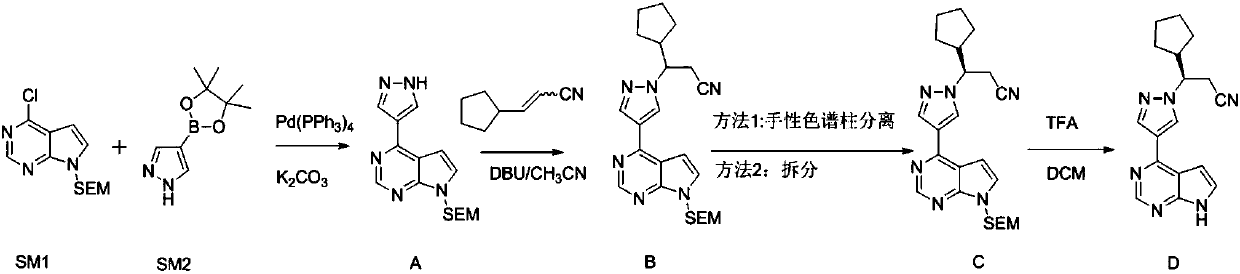
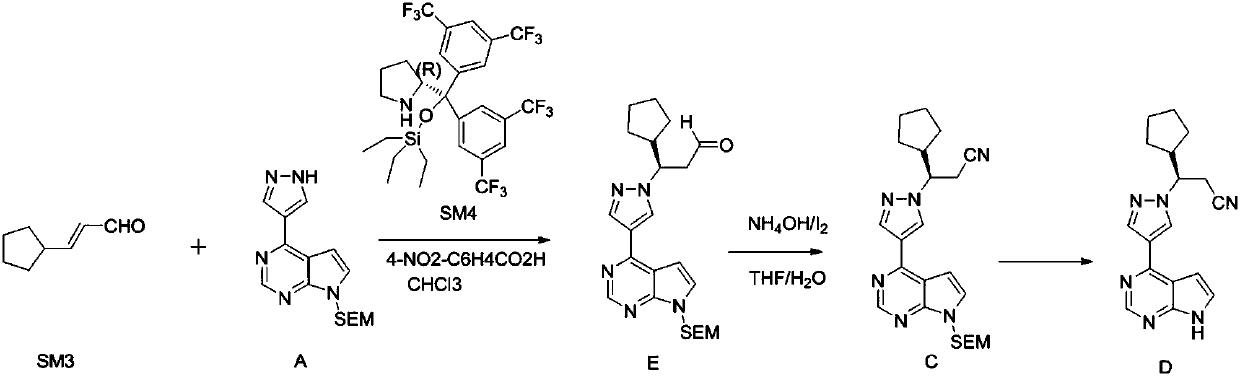
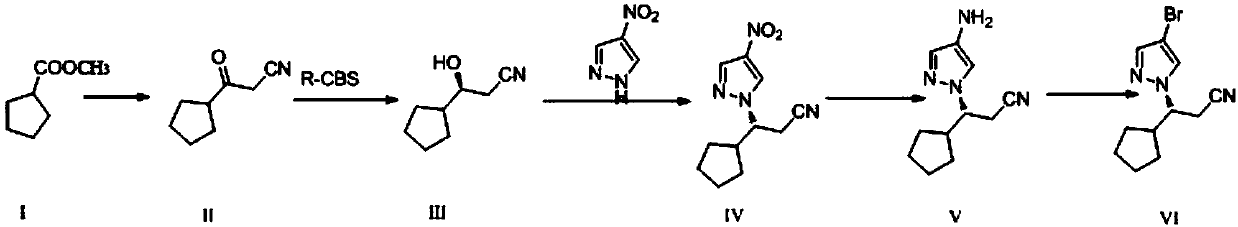
[0029] A preparation method of ruxolitinib intermediate (3R)-3-(4-bromo-1H-pyrazol-1-yl)-cyclopentylpropionitrile, which comprises the following steps:
[0030] (1) Synthesis of 3-oxo-3-cyclopentylpropionitrile (II): Methyl cyclopentylformate reacts with CH under strongly basic conditions 3 CN reaction makes 3-oxo-3-cyclopentylpropionitrile (II);
[0031] (2) Synthesis of (S)-3-cyclopentyl-3-hydroxypropionitrile (III): the 3-oxo-3-cyclopentyl obtained in step (1) is synthesized with chiral borane reagent R-CBS Reduction of propionitrile into (S)-3-cyclopentyl-3-hydroxypropionitrile (III);
[0032] (3) Synthesis of (3R)-3-(4-nitro-1H-pyrazol-1-yl)-cyclopentyl propionitrile (IV): (S)-3- Cyclopentyl-3-hydroxypropionitrile (III) and 4-nitropyrazole were reacted by Mitsunobu to give (3R)-3-(4-nitro-1H-pyrazol-1-yl)-cyclopentylpropane Nitrile (IV);
[0033] (4) Synthesis of (3R)-3-(4-amino-1H-pyrazol-1-yl)-cyclopentylpropionitrile (V): (3R)-3-( Reduction of 4-nitro-1H-pyrazol-1...
Embodiment 1
[0055] 1.1 Synthesis of 3-oxo-3-cyclopentylpropionitrile (II)
[0056] Add NaH (2.75g, 68.7mmol, content 60%) and 20mlTHF in the there-necked flask of 200ml, heat up to 70-75 ℃, dropwise add cyclopentyl formate methyl ester (8.00g, 62.4mmol) under this condition Anhydrous acetonitrile (15ml) solution, control the temperature of the system at 70-75°C, react for 15h, then cool to room temperature, add 50ml of ethyl acetate and 1N HCl solution, control the system pH=2-3, extract and separate the water layer It was extracted three times with ethyl acetate, the organic layers were combined, dried and rotary evaporated to obtain 2.42 g of yellow oil (compound II), with a yield of 91.2%. NMR analysis:
[0057] 1 H-NMR (400MHz, DMSO-d6): 4.09 (2H, s), 3.01 (1H, m), 1.90 (8H, m).
[0058] 1.2 Synthesis of (S)-3-cyclopentyl-3-hydroxypropionitrile (III)
[0059] Under nitrogen protection, 150mlTHF, R-CBS (1.0M toluene solution, 4.35ml, 4.35mmol) and borane dimethyl sulfide (2.0M THF ...
Embodiment 2
[0075] 2.1 Synthesis of 3-oxo-3-cyclopentylpropionitrile (II)
[0076] Add NaH (2.75g, 68.7mmol, content 60%) and 20mlTHF in a 200ml three-necked flask, heat up to 60-65°C, dropwise add cyclopentyl formate methyl ester (8.00g, 62.4mmol) under this condition Anhydrous acetonitrile (15ml) solution, control the temperature of the system at 60-65°C, react for 20h, then cool to room temperature, add 50ml of ethyl acetate and 1N HCl solution, control the system pH=4-5, extract and separate the water layer Then it was extracted three times with ethyl acetate, the organic layers were combined, dried and rotary evaporated to obtain 2.25 g of a yellow oil with a yield of 84.90%.
[0077] 2.2 Synthesis of (S)-3-cyclopentyl-3-hydroxypropionitrile (III)
[0078]Under nitrogen protection, 150mlTHF, R-CBS (1.0M toluene solution, 4.35ml, 4.35mmol) and borane dimethyl sulfide (2.0M THF solution, 33ml, 66mmol) were added to a 500ml three-necked flask, and the reaction temperature was cooled to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com