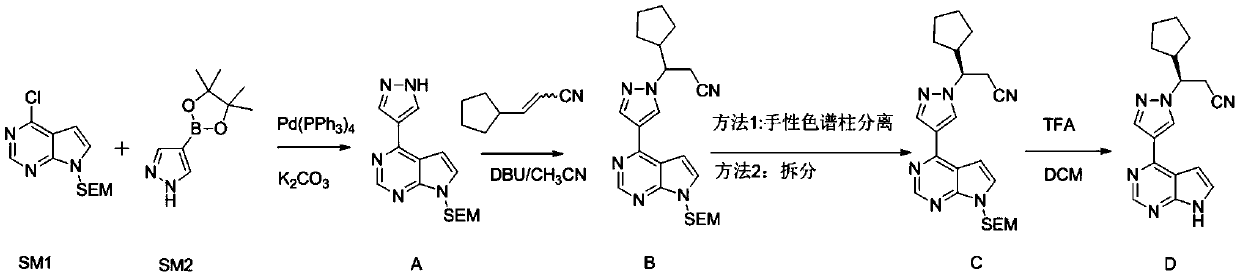
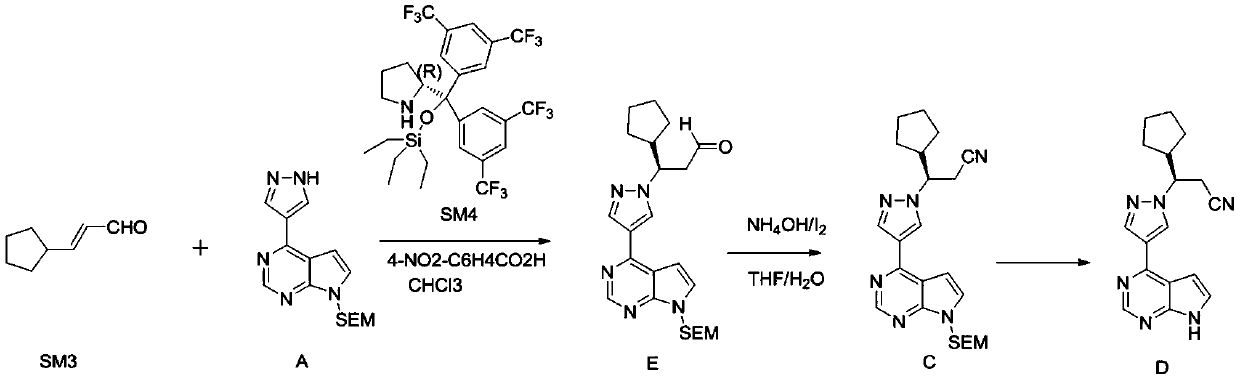
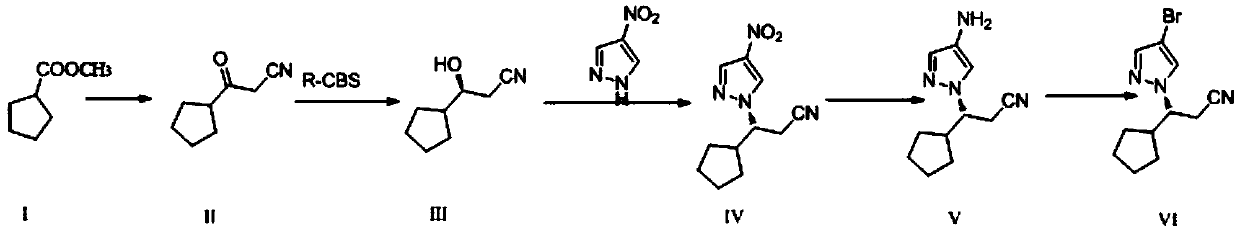
The preparation method of ruxolitinib intermediate (3r)-3-(4-bromo-1h-pyrazol-1-yl)-cyclopentylpropionitrile
A technology of cyclopentyl propionitrile and cyclopentyl propionitrile is applied in the field of preparation of ruxolitinib intermediate-3--cyclopentyl propionitrile, and can solve the problem of high cost and many impurities in API isomers. , the ee value of the intermediate is not high enough, and the effect of mild reaction conditions, good stereoselectivity and high stereoselectivity is achieved.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] A preparation method of ruxolitinib intermediate (3R)-3-(4-bromo-1H-pyrazol-1-yl)-cyclopentylpropionitrile, which comprises the following steps:
[0030] (1) Synthesis of 3-oxo-3-cyclopentylpropionitrile (II): Methyl cyclopentylformate reacts with CH under strongly basic conditions 3 CN reaction makes 3-oxo-3-cyclopentylpropionitrile (II);
[0031] (2) Synthesis of (S)-3-cyclopentyl-3-hydroxypropionitrile (III): the 3-oxo-3-cyclopentyl obtained in step (1) is synthesized with chiral borane reagent R-CBS Reduction of propionitrile into (S)-3-cyclopentyl-3-hydroxypropionitrile (III);
[0032] (3) Synthesis of (3R)-3-(4-nitro-1H-pyrazol-1-yl)-cyclopentyl propionitrile (IV): (S)-3- Cyclopentyl-3-hydroxypropionitrile (III) and 4-nitropyrazole were reacted by Mitsunobu to give (3R)-3-(4-nitro-1H-pyrazol-1-yl)-cyclopentylpropane Nitrile (IV);
[0033] (4) Synthesis of (3R)-3-(4-amino-1H-pyrazol-1-yl)-cyclopentylpropionitrile (V): (3R)-3-( Reduction of 4-nitro-1H-pyrazol-1...
Embodiment 1
[0055] 1.1 Synthesis of 3-oxo-3-cyclopentylpropionitrile (II)
[0056] Add NaH (2.75g, 68.7mmol, content 60%) and 20mlTHF in the there-necked flask of 200ml, heat up to 70-75 ℃, dropwise add cyclopentyl formate methyl ester (8.00g, 62.4mmol) under this condition Anhydrous acetonitrile (15ml) solution, control the temperature of the system at 70-75°C, react for 15h, then cool to room temperature, add 50ml of ethyl acetate and 1N HCl solution, control the system pH=2-3, extract and separate the water layer It was extracted three times with ethyl acetate, the organic layers were combined, dried and rotary evaporated to obtain 2.42 g of yellow oil (compound II), with a yield of 91.2%. NMR analysis:
[0057] 1 H-NMR (400MHz, DMSO-d6): 4.09 (2H, s), 3.01 (1H, m), 1.90 (8H, m).
[0058] 1.2 Synthesis of (S)-3-cyclopentyl-3-hydroxypropionitrile (III)
[0059] Under nitrogen protection, 150mlTHF, R-CBS (1.0M toluene solution, 4.35ml, 4.35mmol) and borane dimethyl sulfide (2.0M THF ...
Embodiment 2
[0075] 2.1 Synthesis of 3-oxo-3-cyclopentylpropionitrile (II)
[0076] Add NaH (2.75g, 68.7mmol, content 60%) and 20mlTHF in the there-necked flask of 200ml, heat up to 60-65 ℃, dropwise add cyclopentyl formate methyl ester (8.00g, 62.4mmol) under this condition Anhydrous acetonitrile (15ml) solution, control the temperature of the system at 60-65°C, react for 20h, then cool to room temperature, add 50ml of ethyl acetate and 1N HCl solution, control the system pH=4-5, extract and separate the water layer Then it was extracted three times with ethyl acetate, the organic layers were combined, dried and rotary evaporated to obtain 2.25 g of a yellow oil with a yield of 84.90%.
[0077] 2.2 Synthesis of (S)-3-cyclopentyl-3-hydroxypropionitrile (III)
[0078]Under nitrogen protection, 150mlTHF, R-CBS (1.0M toluene solution, 4.35ml, 4.35mmol) and borane dimethyl sulfide (2.0M THF solution, 33ml, 66mmol) were added to a 500ml three-necked flask, and the reaction temperature was cool...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com