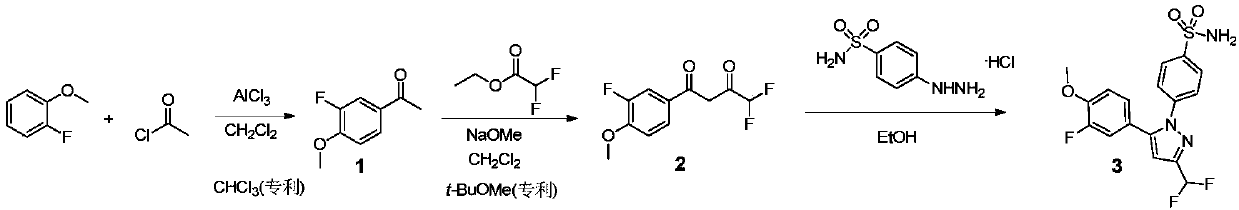
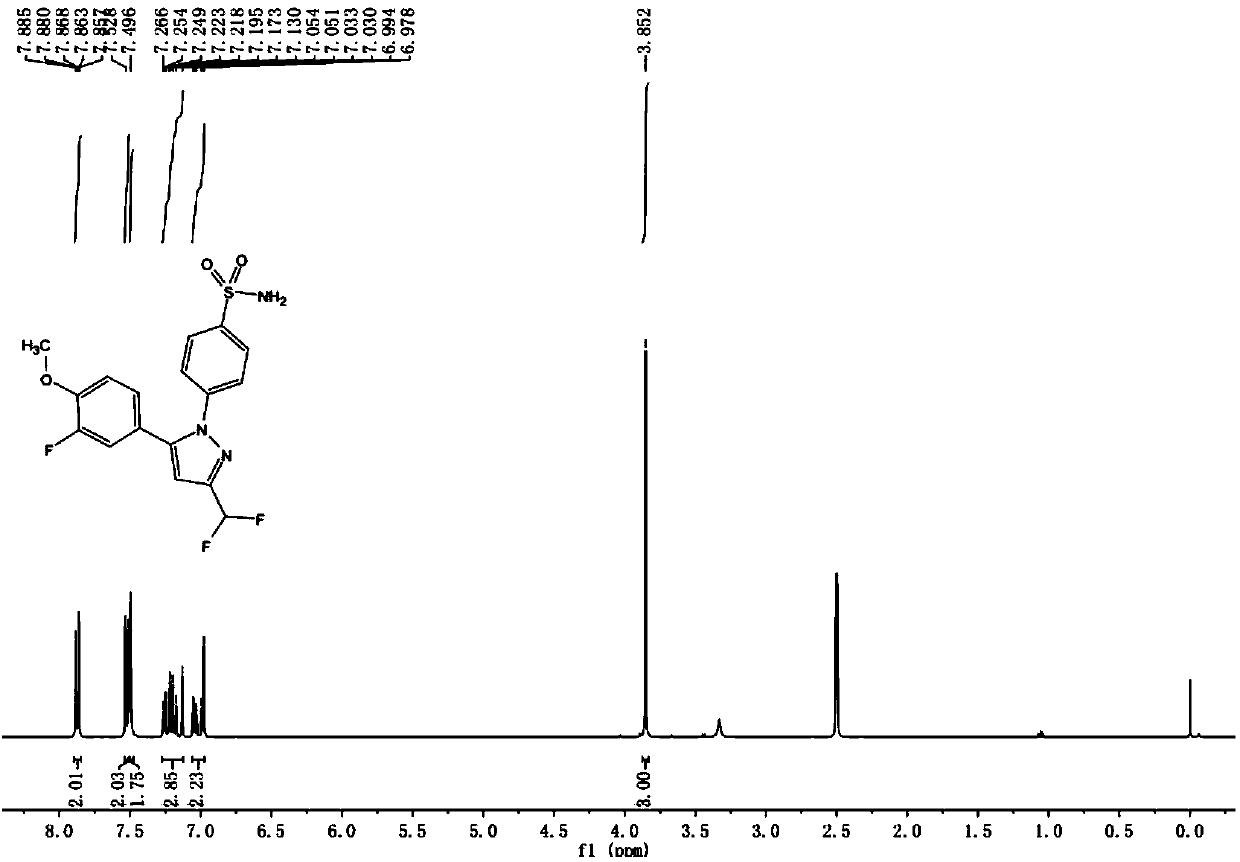
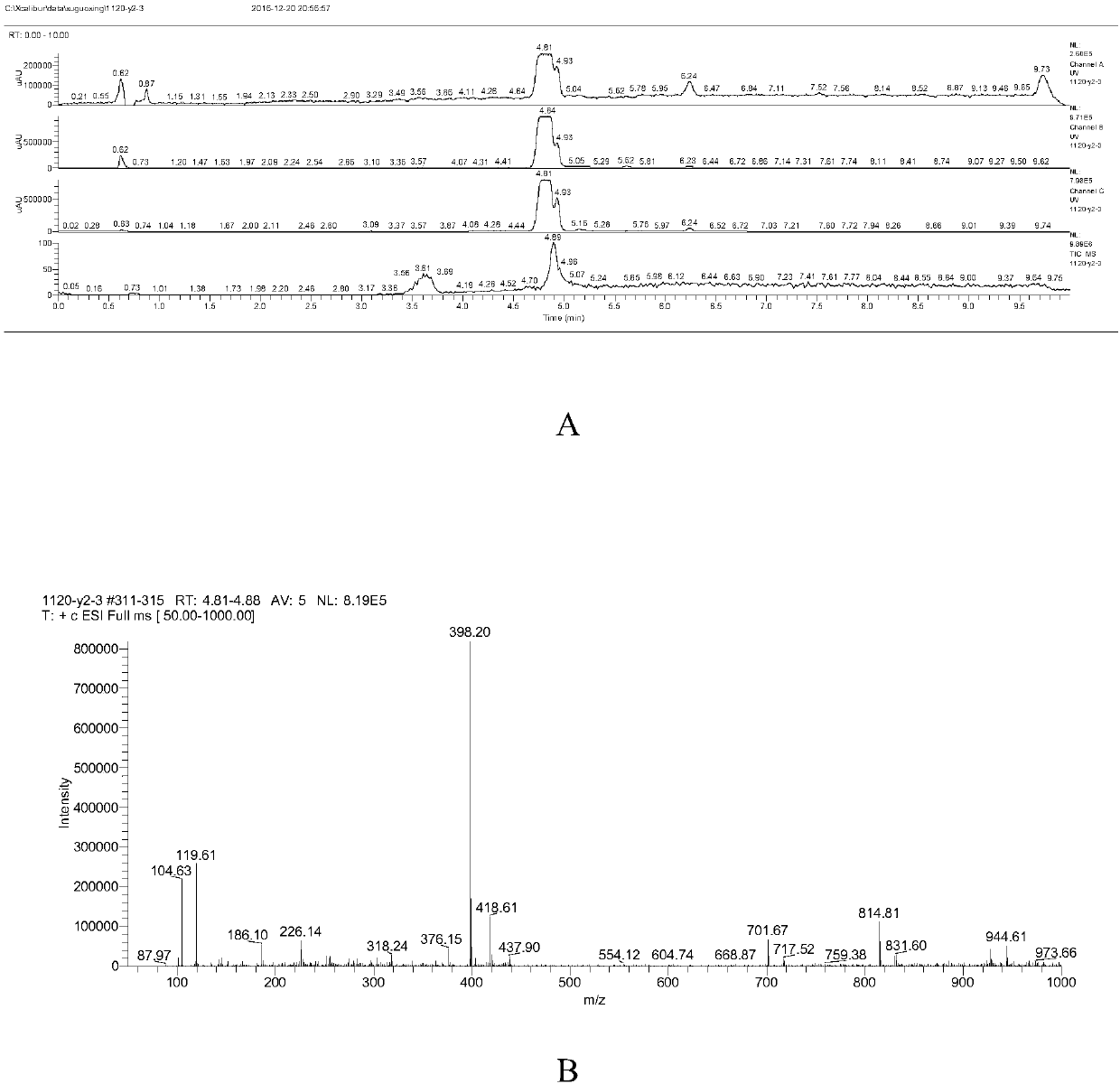
Preparation method of Deracoxib
A cooxib and reaction technology, which is applied in the field of preparation of diracoxib, can solve the problems of a large amount of solvent, high production cost and high price, and achieves the effects of simple operation, mild conditions and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The preparation of embodiment 1 deracoxib
[0041] Step 1 (Step 1): Preparation of 3-fluoro-4-methoxyacetophenone (compound 1): under ice-water bath conditions, put 6.38g (48mmol) aluminum trichloride and 40ml dichloromethane into 100ml circle In the bottom flask, after stirring and dissolving, add 4.68g (60mmol) of acetyl chloride dropwise, maintain the temperature at 5-10°C, stir at 5°C for 10 minutes, and then add 5.04g (40mmol) of 2-fluoroanisole dropwise. The mixture was stirred at 5°C for 1 hour and then poured into 100ml of ice water, the aqueous layer was extracted with dichloromethane (2×30ml), the combined organic phases were washed with water (2×30ml), dried over anhydrous magnesium sulfate, suction filtered, and washed with petroleum ether The product was recrystallized from ethyl acetate with a yield of 92.4%.
[0042] Step 2 (Step 2): Preparation of 4,4-bisfluoro-1-(3-fluoro-4-methoxyphenyl)-1,3-butanedione (compound 2): 1.79g (14.4mmol ) Ethyl difluoroa...
experiment example 1
[0049] Experimental Example 1 Parameter optimization for the preparation of deracoxib
[0050] 1. Optimization of preparation parameters of 3-fluoro-4-methoxyacetophenone
[0051] Operation: Put aluminum trichloride and dichloromethane into a round-bottomed flask in an ice-water bath, stir to dissolve, add acetyl chloride dropwise, keep the temperature at 0-10°C and stir for 10 minutes, then add 2-fluoro Anisole, the mixture continued to stir at this temperature for 0.5-2 hours and then poured into ice water, the water layer was extracted with dichloromethane, the combined organic phases were washed with water, dried with anhydrous magnesium sulfate, suction filtered, and washed with petroleum ether and ethyl acetate The ester was recrystallized to obtain the product.
[0052] The different reaction parameters are shown in Table 1. The amount of DCM (ml) is 6-10 times the amount of 2-fluoroanisole mass (g) respectively (other reaction parameters are the same as in Example 1),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com