A cell imaging method
A cell and imaging technology, applied in the direction of material excitation analysis, fluorescence/phosphorescence, instruments, etc., can solve the problems of weakening fluorescence intensity, occupying excitation and emission channels, time-consuming and labor-intensive, etc., to reduce the influence of cells and increase selectivity , The effect of simplifying the experimental operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Example 1: LSCM detection and spectral scanning of unlabeled cells.
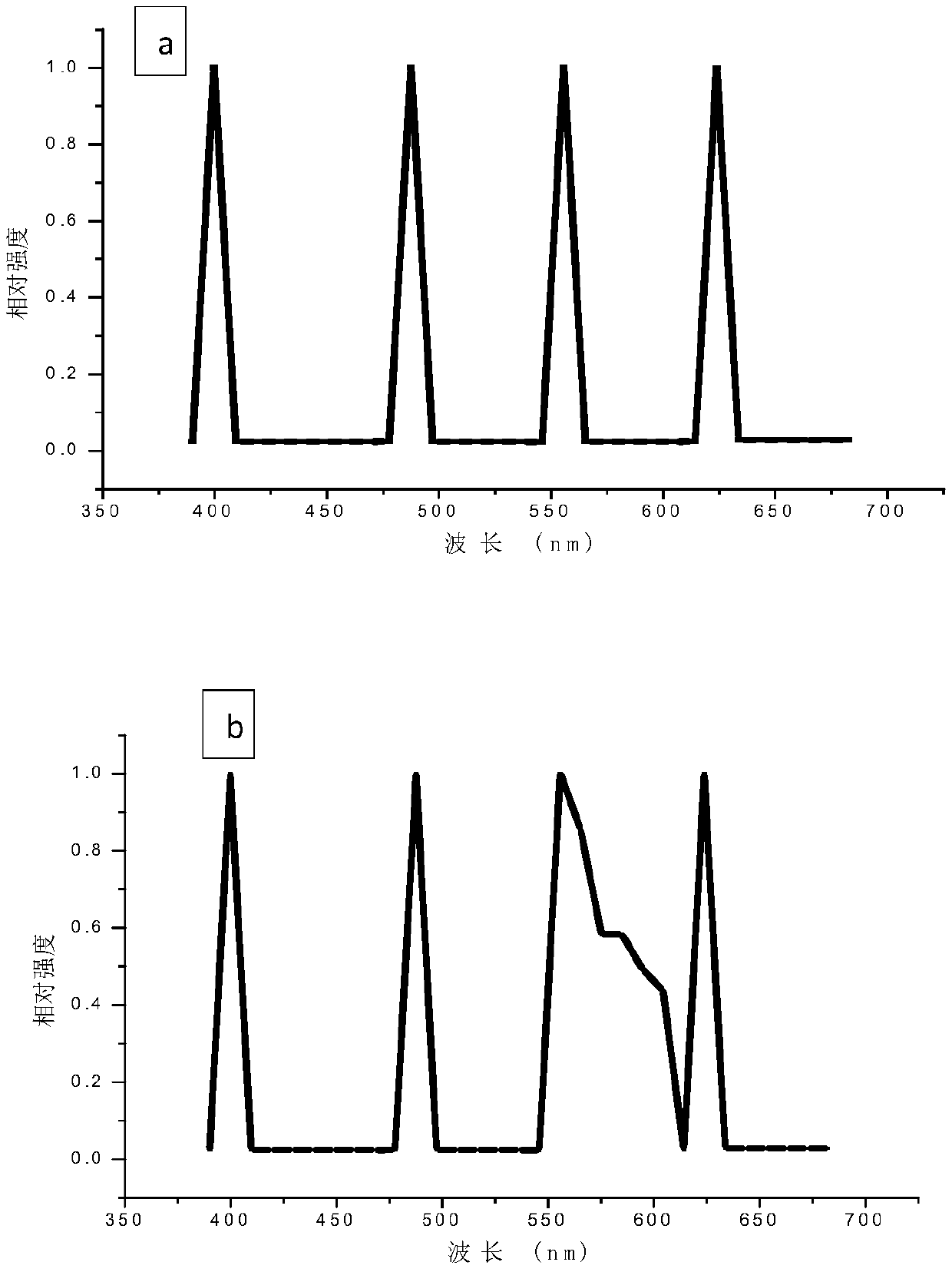
[0068] A549 cells were planted in a confocal glass dish, and the cells were adhered to the appropriate density and placed under an LSCM microscope. In the process of spectral scanning, four sets of excitation light source channels of LSCM are turned on, and lasers of four wavelengths are emitted: UV-405nm, Ar-488nm, Kr-561nm, HeNe-633nm, the detection wavelength range is set to 400nm to 680nm, and the step distance is 5nm. Plot the intensity-wavelength curve. Test results such as figure 1 Shown in (a) is the emission spectrum of A549 cells in the range of receiving wavelengths. There is a sharp peak at 405, 488, 561, and 633nm on the spectrum of A549 cells, and the peak width is only about 10nm. These peaks are all at the position of the excitation wavelength.
Embodiment 2
[0069] Example 2: LSCM detection and spectral scanning of cells labeled with DiI fluorescence.
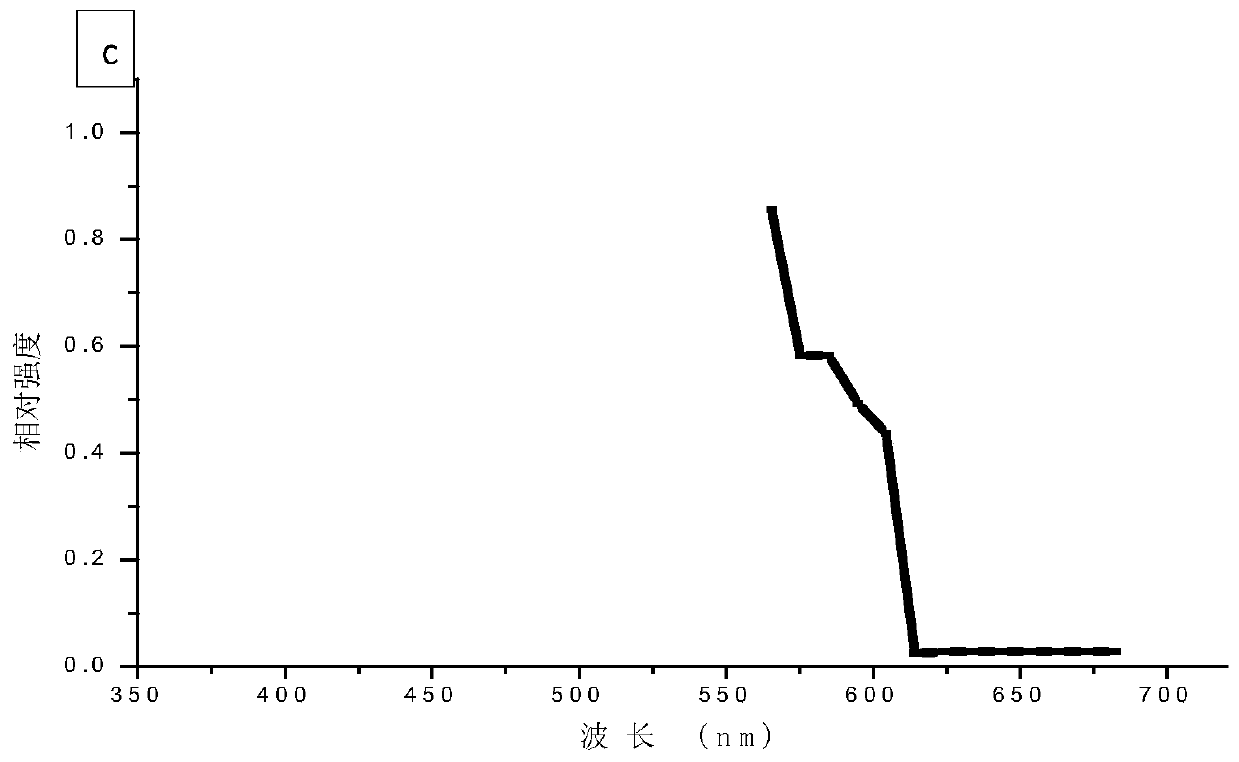
[0070] A549 cells were planted in a confocal glass dish, and the cells were adhered to the wall and cultured to a suitable density. The cell membrane was fluorescently labeled with DiI, and then placed under an LSCM microscope for detection. DiI solution was used as the control group. In the process of spectral scanning, four sets of excitation light source channels of LSCM are turned on, and lasers of four wavelengths are emitted: UV-405nm, Ar-488nm, Kr-561nm, HeNe-633nm, and the detection wavelength range is set to 400nm to 680nm, and the step distance is 5nm. Plot the intensity-wavelength curve. Test results such as figure 1 Shown in (b) and (c) are the emission spectrum diagrams of the DiI fluorescently labeled cells and the DiI solution in the receiving wavelength range of 400-680 nm, respectively. Where (b) represents DiI fluorescently labeled cells, and (c) represents DiI...
Embodiment 3
[0072] Example 3: Cells labeled with DiI and Hoechst3332 fluorescence are detected by LSCM
[0073] A549 cells were planted in a confocal glass dish. After the cells adhered to the wall and cultured to a suitable density, the cell membrane was fluorescently labeled with DiI, and the cell nucleus was labeled with Hoechst3332, and then placed under an LSCM microscope for detection.
[0074] image 3 For cells labeled with DiI and Hoechst33342 fluorescence, LSCM was used for planar imaging, where: (b) is the partially enlarged image of (a), and (c) is the xzy longitudinal section image of (b). As shown in the figure, the SRL channel can clearly image the cells and has co-localization with DiI.
[0075] Figure 4 It is the image of the cell samples of each layer scanned continuously along the z-axis under LSCM, in which: (a) Hoechst33342 fluorescence channel, (b) DiI fluorescence channel, (c) SRL channel, (d) overlay of a, b, c. As shown in the figure, both the SRL channel and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com