Carborane-perylene diimide derivative and synthesis method thereof, sensing array based on derivative and preparation method and application of sensor array
A perylene diimide and sensing array technology, which is applied in the field of small molecule fluorescent sensing thin film materials, can solve the problems of complex synthesis methods of fluorescent components, large differences in excitation and emission characteristics, and increasing difficulty in the preparation process, and achieves practical application. High performance, eliminate interference, and ensure the effect of simplicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
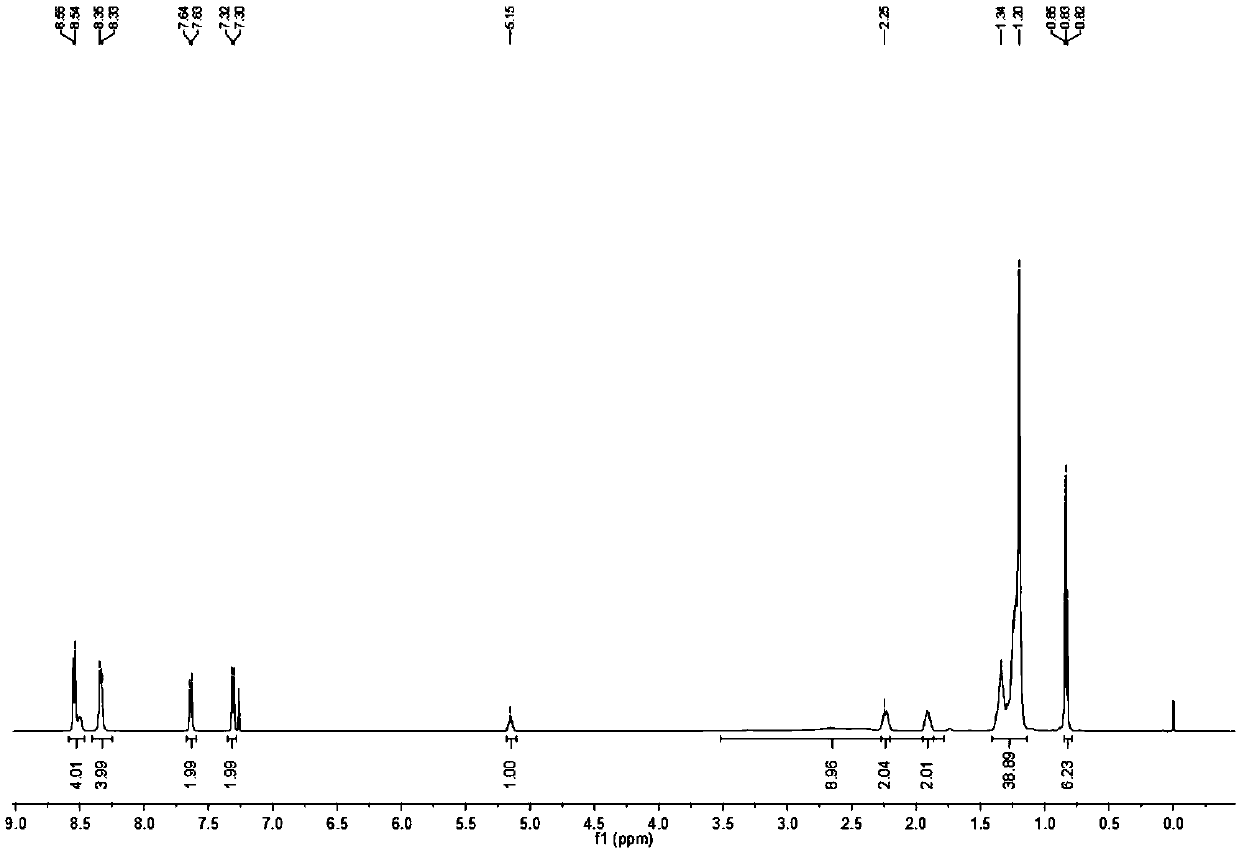
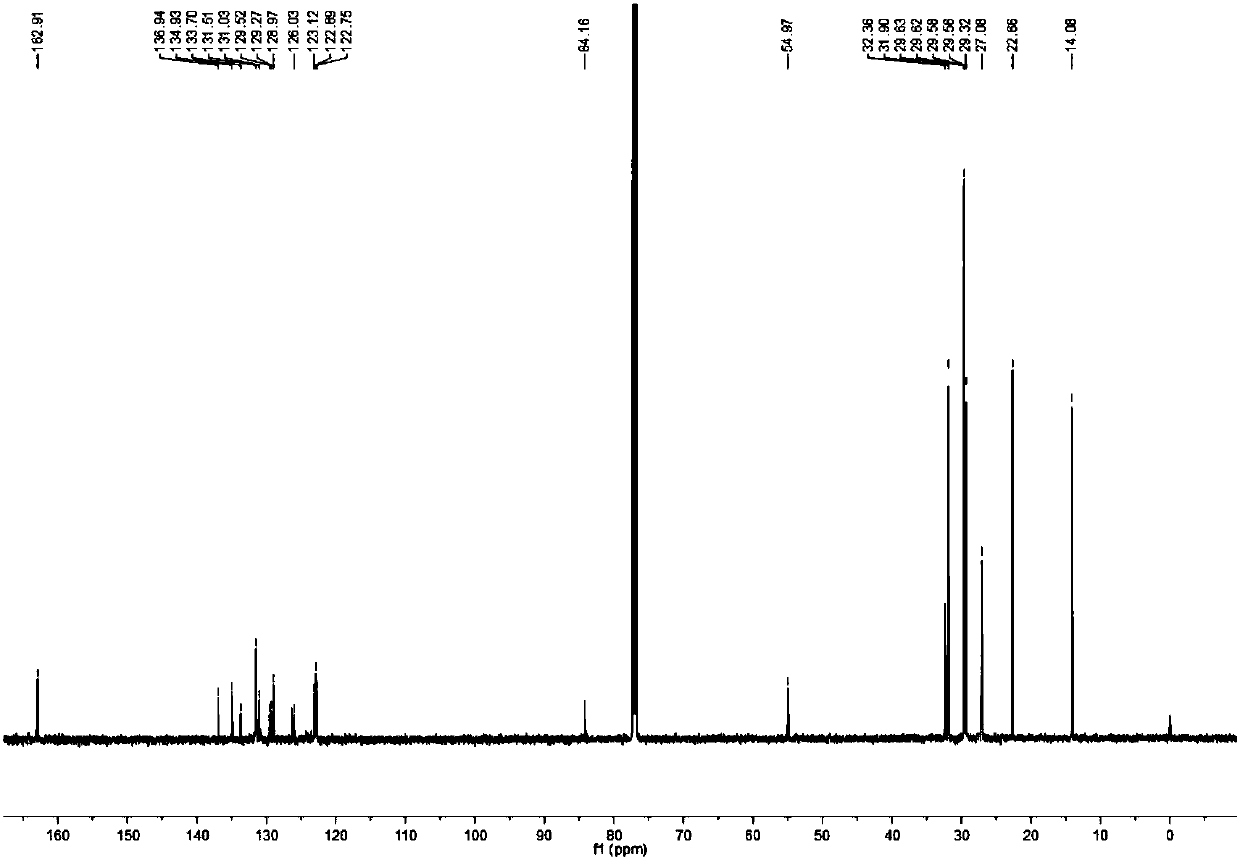
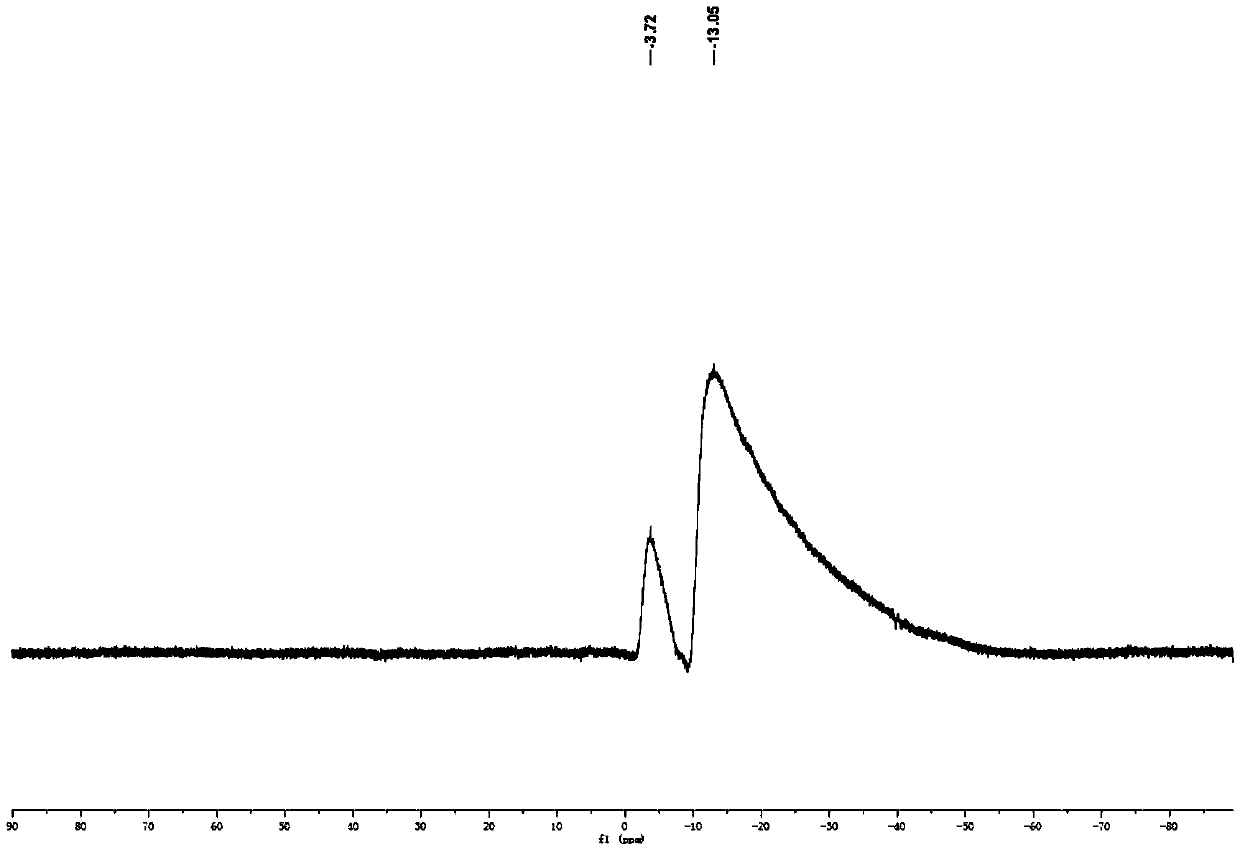
[0069] Synthesis of carborane-perylene diimide derivatives (in this example, n=9)
[0070] Wherein the raw material selects p-iodoaniline, p-ethynyl aniline, and the alkyl ketone selects 12-tritricone;
[0071] (1) Synthesis of compound 1
[0072] Take by weighing 2.19g p-iodoaniline, 0.58g Pd(PPh 3 ) 4 , 0.095g CuI and 1.17g p-ethynylaniline were placed in a 100mL two-necked bottle. Under nitrogen protection, 10 mL of triethylamine and 40 mL of tetrahydrofuran were sequentially added to the reaction system, heated to 70° C., stirred for 12 hours, cooled to room temperature, and spin-dried. A brown solid was obtained by column chromatography using dichloromethane:petroleum ether (1:1) as the eluent. The resulting solid was dried under vacuum at 50°C. Its reaction equation is as follows:
[0073]
[0074] (2) Synthesis of Compound 2
[0075] Weigh 3.39g of 12-docotriconone and 1.38g of hydroxylamine hydrochloride in a 100mL single-necked bottle, add 10mL of pyridine a...
Embodiment 2
[0091] Synthesis of carborane-perylene diimide derivatives (n=5 in this embodiment)
[0092] Wherein the raw material selects p-iodoaniline, p-ethynyl aniline, and the alkyl ketone selects 8-pentadecanone;
[0093] (1) Synthesis of compound 1
[0094] Take by weighing 2.19g p-iodoaniline, 0.58g Pd(PPh 3 ) 4 , 0.095g CuI and 1.17g p-ethynylaniline were placed in a 100mL two-necked bottle. Under nitrogen protection, 10 mL of triethylamine and 40 mL of tetrahydrofuran were sequentially added to the reaction system, heated to 70° C., stirred for 12 hours, cooled to room temperature, and spin-dried. A brown solid was obtained by column chromatography using dichloromethane:petroleum ether (1:1) as the eluent. The resulting solid was dried under vacuum at 50°C. Its reaction equation is as follows:
[0095]
[0096] (2) Synthesis of compound 2
[0097] Weigh 2.26g of 8-pentadecanone and 1.38g of hydroxylamine hydrochloride in a 100mL single-necked bottle, add 10mL of pyridin...
Embodiment 3
[0112] Synthesis of Carborane-Perylene Diimide Derivatives (n=9 in this example)
[0113] Wherein the raw material selects m-iodoaniline, m-ethynyl aniline, and the alkyl ketone selects 12-tritricone;
[0114] (1) Synthesis of compound 1
[0115] Weigh 2.19g m-iodoaniline, 0.58g Pd(PPh 3 ) 4 , 0.095g CuI and 1.17g m-ethynylaniline were placed in a 100mL two-necked flask. Under nitrogen protection, 10 mL of triethylamine and 40 mL of tetrahydrofuran were sequentially added to the reaction system, heated to 60° C., stirred for 8 hours, cooled to room temperature, and spin-dried. A brown solid was obtained by column chromatography using dichloromethane:petroleum ether (1:1) as the eluent. The resulting solid was dried under vacuum at 40°C. Its reaction equation is as follows:
[0116]
[0117] (2) Synthesis of compound 2
[0118] Weigh 3.39g of 12-docotriconone and 1.38g of hydroxylamine hydrochloride in a 100mL single-necked bottle, add 10mL of pyridine and 22mL of eth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com